Study finds stool transplants more effective than antibiotics for treating recurring, life-threatening gut infections
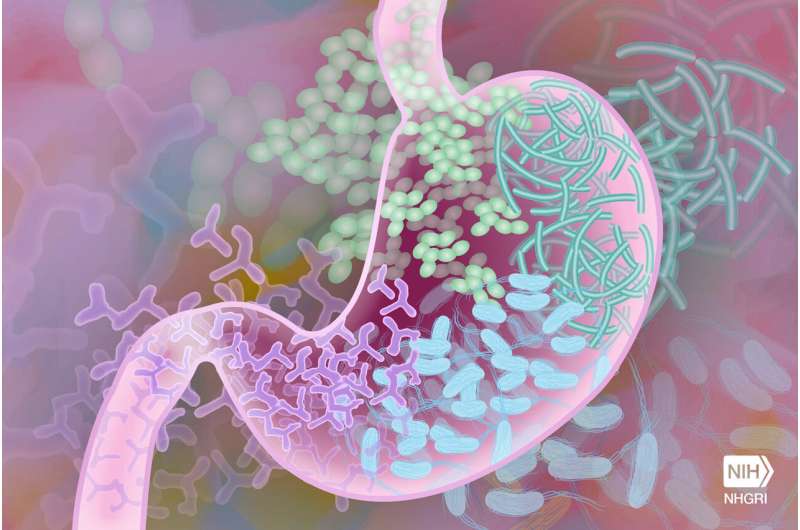
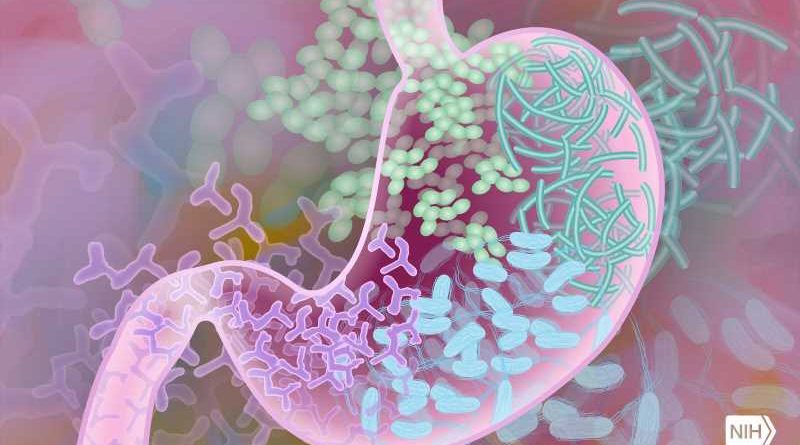
A new study, published in the Cochrane Database of Systematic Reviews and led by an Upstate Medical University professor, has found that compared with standard antibiotic treatment, stool transplantation can increase the number of people recovering from Clostridioides difficile (C. diff) infection, a condition that causes potentially life-threatening diarrhea. Within the study, 77% of people who received a stool transplant did not experience reinfection within eight weeks, compared to 40% of those who received antibiotics alone.
C. diff is a bacterium that can cause life-threatening diarrheal illness in individuals with an unhealthy mixture of gut bacteria, known as dysbiosis. The most common cause of dysbiosis is treatment with antibiotics, and while antibiotics can be very effective against bacterial infections, they can also harm the beneficial bacteria colonizing the gut, known as the intestinal microbiome. Usually this ecosystem of “good” bacteria recovers quickly, but occasionally “bad” species like C. diff take over and cause serious diarrhea.
The standard treatment of C. diff infection includes antibiotics, which may further exacerbate dysbiosis. This can lead to a vicious cycle of brief treatment effect followed by a recurrent infection. This happens in nearly a third of infected individuals. According to the CDC, every year there are around a quarter of a million C. diff infections in the US alone, causing approximately 12,000 fatalities.
Transplanting healthy donor stool into a gut with dysbiosis is intended to balance the gut microbes and reestablish a healthy microbiome, thus significantly reducing the risk of C. diff recurring. Stool donation operates much the same way as blood donation. Donors are screened for diseases and infections before they can donate their stool. The stool can be transplanted via colonoscopy, nasogastric or nasoduodenal tube, enema or via a capsule. The US Food and Drug Administration has recently approved a stool transplant product for prevention of recurrence of C. diff that can be administered as enema.
The new Cochrane Review, led by pediatric gastroenterologist Aamer Imdad, MBBS, examined data from six clinical trials with a total of 320 adults that assessed the efficacy and safety of stool transplantation for the treatment of repeated C. diff infection. Two studies were conducted in Denmark, and one each in the Netherlands, Italy, Canada, and the United States. Most of the included studies compared stool transplantation with a standard antibiotic treatment using vancomycin, which is commonly used for this kind of infection.
The review found that stool transplantation leads to a larger increase in resolution of repeated infections of C. diff than other treatments studied, as well as a decrease in side effects when compared with standard treatment using antibiotics.
“After a person with a C. diff infection gets treated with antibiotics, there is about a 25% chance that they will have another episode of C. diff infection in the next 8 weeks,” Imdad said. “The risk of recurrence increases to about 40% with the second episode and to nearly 60% with the third episode. So, once you are in this cycle, it gets more and more difficult to break out of it. Stool transplants can reverse the dysbiosis and thus decrease the risk of recurrence of the disease.”
A second Cochrane Review, also led by Dr. Imdad, looks at the use of stool transplants for the treatment of inflammatory bowel disease (IBD), a term mainly used to describe two conditions: ulcerative colitis and Crohn’s disease. The review shows promising results for ulcerative colitis; however, the data is not conclusive yet. Results for Crohn’s disease are even less conclusive. More research will be required before stool transplants can be considered for the treatment of IBD.
More information:
Fecal microbiota transplantation for the treatment of recurrent Clostridioidesdifficile (Clostridium difficile), Cochrane Database of Systematic Reviews (2023). DOI: 10.1002/14651858.CD013871.pub2
Journal information:
Cochrane Library
Source: Read Full Article