A reference tissue atlas for the human kidney
A team of researchers including Jens Hansen, Rachel Sealfon, Rajastree Menon, and colleagues of the Kidney Precision Medicine Project, built on an existing specific human kidney tissue atlas relevant to health care at single-cell resolution. They advanced the atlas by integrating a reference map of cells, pathways and genes to the existing product. These additions involved unaffected areas of nephrectomy tissues and undiseased human biopsies. The team targeted single-cell nucleus transcriptomics including sub-segmental laser microdissection transcriptomics and proteomics to identify these genes, pathways and cells. The outcomes described the cellular-level functional organization of the kidney relative to its physiological function and then linked cell subtypes to genes, proteins, metabolites and pathways. The messenger RNA levels along the nephron agreed well with the sub-segmental physiological activity to provide a reference atlas as a framework to classify kidney disease with multiple molecular mechanisms underlying convergent clinical phenotypes.
A reference human kidney atlas
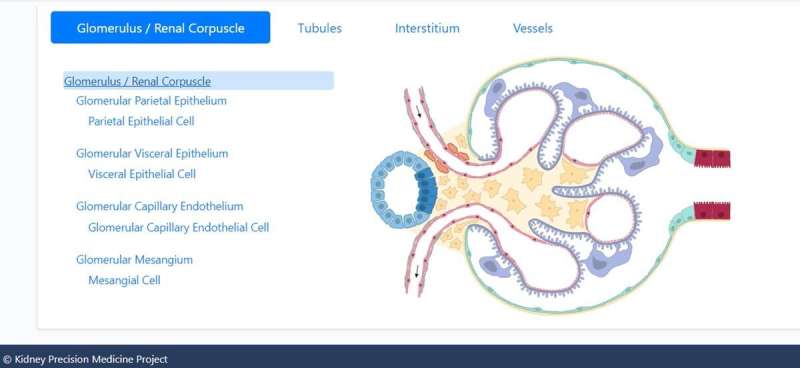
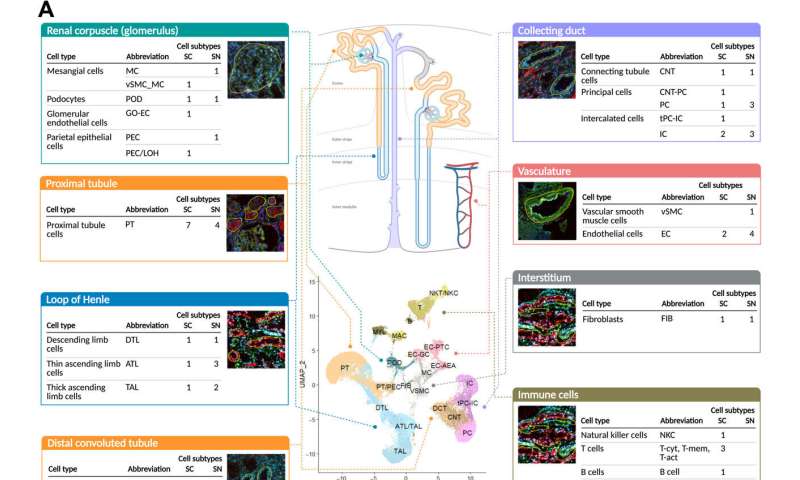
The kidney maintains diverse cell populations and is critical to facilitate physiological homeostasis by regulating fluid and electrolyte balance, osmolarity and pH. The nephron is the basic unit of organization in the kidney and is embedded in the interstitium, which contains between 210,000 and 2.7 million nephrons. Nephrons and the interstitium maintain multiple cell types to form blood vessels and capillaries, including endothelial cells and vascular smooth muscle cells, as well as a range of immune cells. Researchers have maintained sustained efforts to develop structure-function endo-phenotype relationships within the kidney tissue to understand the physiology and pathophysiology of the organ. In this work, Hansen et al studied 56 adult human subjects, and analyzed 80,289 single cells. The scientists constructed maps of diverse cell types in the kidney as well as the molecular entities and functional pathways in these cell types to form a unique reference human kidney atlas. The data of the study can be downloaded as an interactive cellular atlas to provide a starting point to understand disease states in the kidney and project a new functional context to drive new molecular classifications of kidney disease.
The Kidney Precision Medicine Project (KPMP) Consortium
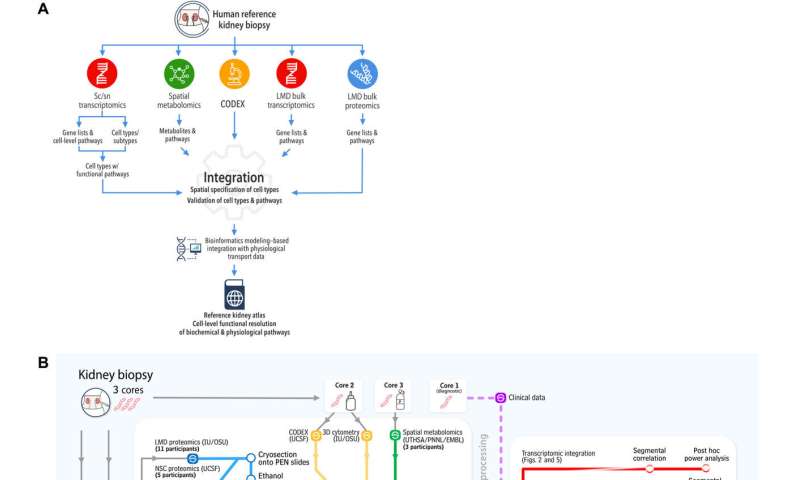
To build the reference atlas, the scientists first conducted a variety of omics arrays and low-throughput imaging experiments at different sites. The specimens used in the work did not show any structural signs of disease, and were obtained from nephrectomies and biopsies of donors and transplant recipients. Based on the shared tissue samples, Hansen et al conducted four transcriptomic, two proteomic, two imaging-based, and one spatial metabolomics tissue interrogation assays. The researchers then integrated multiple transcriptomic interrogation methods to show agreement and technical synergy between the assays. The outcomes confirmed all known major kidney tissue cell types of the nephron and multiple immune cells. The team conducted additional independent methods to improve the atlas framework.
Combining proteomic and transcriptomic assays
Hansen et al performed subsegment-specific protein expression profiles via two different proteomic assays to produce biologically complementary descriptions in the study. The assays identified protein expression in the glomerulus and tubulointerstitium, as well as the proximal tubule. They focused on podocyte/glomerular and proximal tubule cells during the work and sub-segmented into four transcriptomic datasets. Based on clustering datasets, they readily identified groups of genes or proteins associated with the appropriate anatomical regions. The outcomes highlighted the significance of relative expression levels of genes or proteins to indicate the corresponding anatomic region of the kidney. The combination of multiple datasets increased the accuracy of the results. The team further integrated imaging assays to the experiments to arrange different cell subtypes along the nephron and interstitium to document the order in which the regions encountered the glomerular filtrate.
Molecular basis of physiological functions at cellular resolution
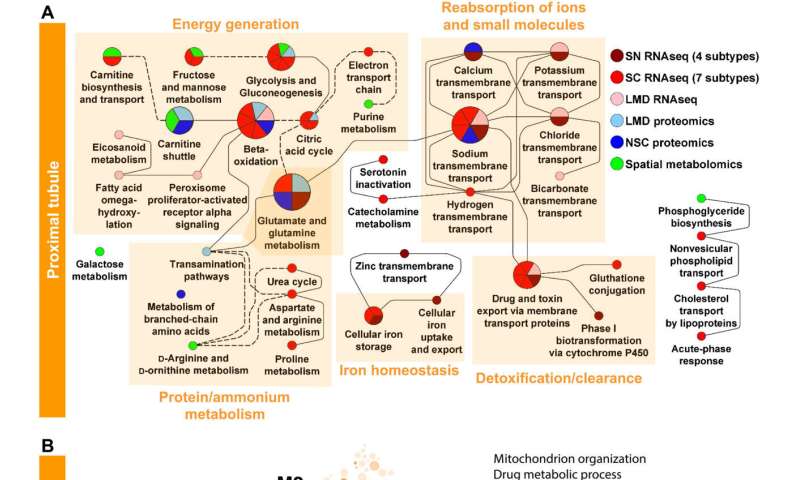
After establishing the consistency across transcriptomics, proteomics and imaging datasets, Hansen et al integrated data to identify cell type-specific functional pathways and network modules. They used individual analyses of single-cell and single-nucleus RNA sequencing datasets by identifying the pathways referred to from the expressed genes. The work facilitated the identification of functional capabilities of different cell types from the kidney. Further outcomes established the accuracy with which they assigned pathways and physiological functions to different cell types. The team subjected the top 300 significant gene and protein markers of each cell type or subtype to dynamic enrichment analysis through molecular biology of the cell ontology analyses. They obtained fewer than 300 significant markers and consequently used them for downstream analyses and mapped their predicted pathways to create a detailed map of pathway activities in all major cell types in the kidney. The outcomes identified many well-known cellular activities for a range of kidney cell types.
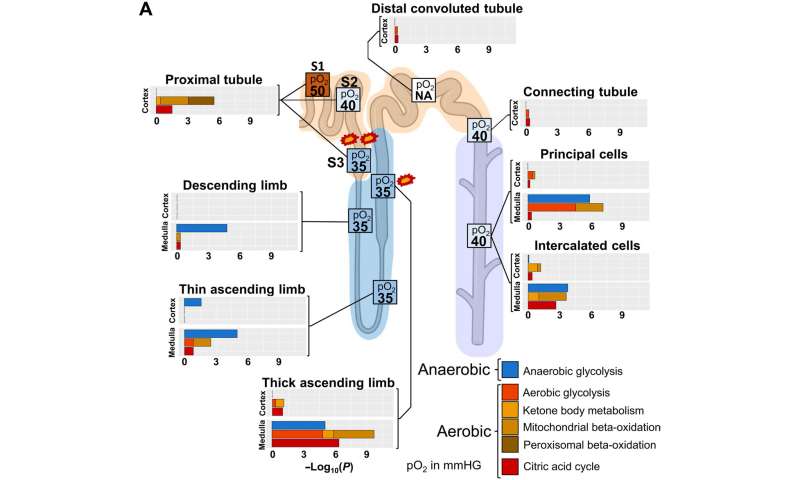
Identifying sites vulnerable to kidney injury
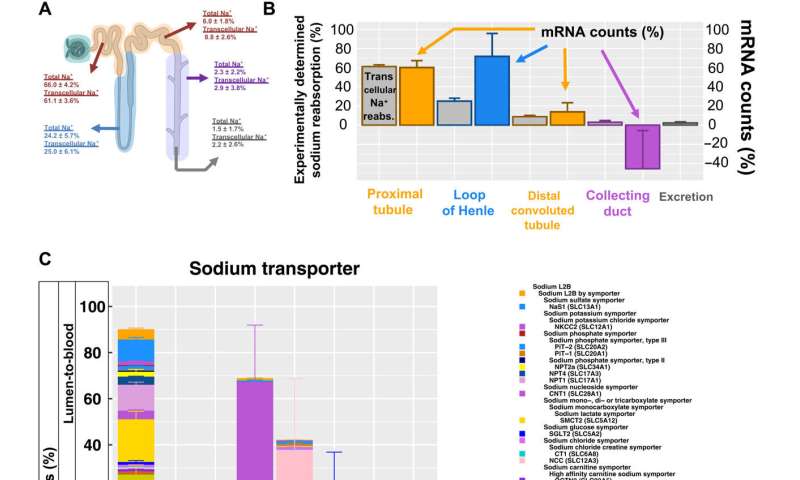
The scientists additionally studied the energy generation pathways in different cells along the renal tubule of the nephron by generating a focused ontology of metabolic pathways. They mapped the expression patterns of different paths involved in aerobic and anaerobic energy production to varying levels of oxygen availability in different parts of the nephron. The comparison allowed them to identify regions with higher susceptibility towards hypoxia-induced kidney injury. Molecular profiles of metabolic pathways in the atlas generally provide a fundamental outlook to understand kidney injury due to hypoxia in a clinical setting. The team conducted studies to determine the percentage of a filtered ion or small molecule reabsorbed in a specific segment of the nephron or excreted in the ureter. Since some nephron segments contain multiple cell types with diverse reabsorption mechanisms, they focused on cell type-specific transport mechanisms. The cell atlas therefore provided a detailed picture of sodium reabsorption in agreement with experimental reabsorption profiles for the first time.
Outlook: Clinical setting
Source: Read Full Article
