Combining epigenetic and metabolic approaches for targeted cancer treatment
Northwestern Medicine scientists have identified a novel vulnerability in a subset of genes commonly mutated in cancer, according to a study recently published in the Journal of Clinical Investigation.
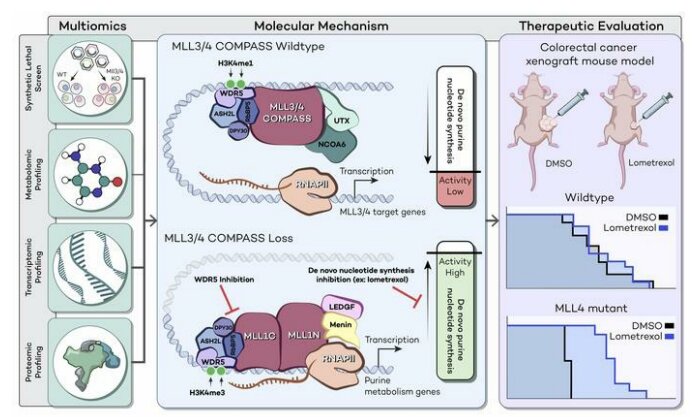
Mutations in a set of genes known as the COMplex of Proteins Associated with Set1 (COMPASS) family, specifically mutations in the genes MLL3 and MLL4, are common in patients with many types of cancer.
To better understand these genes and what role they play in tumor growth, investigators performed a CRISPR screen in embryonic stem cells from mice with both MLL3 and MLL4 deleted. The MLL3 and MLL4 knockout cells appeared to ramp up the production of purine, an essential building block for DNA and RNA, according to the study.
The scientists then treated the MLL3 and MLL4 deleted cells with a purine synthesis inhibitor, which hampered cell growth.
To see if cancers with mutations in these genes could be slowed with a similar tactic, the investigators tested their approach on cells from patients with colorectal cancer, which commonly features mutations in MLL3 and/or MLL4. They found that cancer cells with mutations in MLL3/4 were more sensitive to the purine synthesis inhibitor as compared to cells expressing wild type MLL4, according to the study.
The findings could represent a potential treatment direction for different cancers that share MLL3 or MLL4 mutations, said Ali Shilatifard, Ph.D., the Robert Francis Furchgott Professor and chair of Biochemistry and Molecular Genetics and senior author of the study.
“MLL3 and MLL4 are highly mutated in a large number of solid tumors and hematopoietic malignancies. There’s very little therapy for these cancers and understanding what causes the cells to become cancerous as a result of this mutation is going to be central for the development of targeted therapeutics,” said Shilatifard, who is also the director of the Simpson Querrey Institute for Epigenetics and leader of the Cancer Epigenetics & Nuclear Dynamics Program at Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “Our work is showing that basically the purine metabolism inhibition is one way of treating the cells that have mutations for MLL4.”
Shilatifard said he hopes to move to preclinical studies to further validate the efficacy of the approach. “A large number of tumors don’t respond to immunotherapy. We think that this mixture of immunotherapy with epigenetic therapy and metabolism therapy, that combination could be very powerful,” he said.
Zibo Zhao, Ph.D., research assistant professor of Biochemistry and Molecular Genetics, was first author of the study. Kaixiang Cao, Ph.D., a former postdoctoral fellow in Shilatifard’s lab, was also a co-author of the study.
“We hope our research can support the translation of this to clinical settings and then we could use this as a biomarker to stratify the patients, so they can get more personalized therapy by targeting these mutations in human cancer,” Zhao said.
More information:
Zibo Zhao et al, Therapeutic targeting of metabolic vulnerabilities in cancers with MLL3/4-COMPASS epigenetic regulator mutations, Journal of Clinical Investigation (2023). DOI: 10.1172/JCI169993
Journal information:
Journal of Clinical Investigation
Source: Read Full Article