First in-human study of golodirsen for Duchenne muscular dystrophy shows safety and biologic activity
This first-in-human study of golodirsen showed its long-term safety and biologic activity in patients with Duchenne Muscular Dystrophy (DMD). The approved exon-skipping therapy is designed to enable the production of functional dystrophin proteins, as described in the peer-reviewed journal Nucleic Acid Therapeutics.
Golodirsen was tested in ambulatory patients with exon 53 skip amenable DMD. They were at an age associated with progressive deterioration and declining ambulatory function.
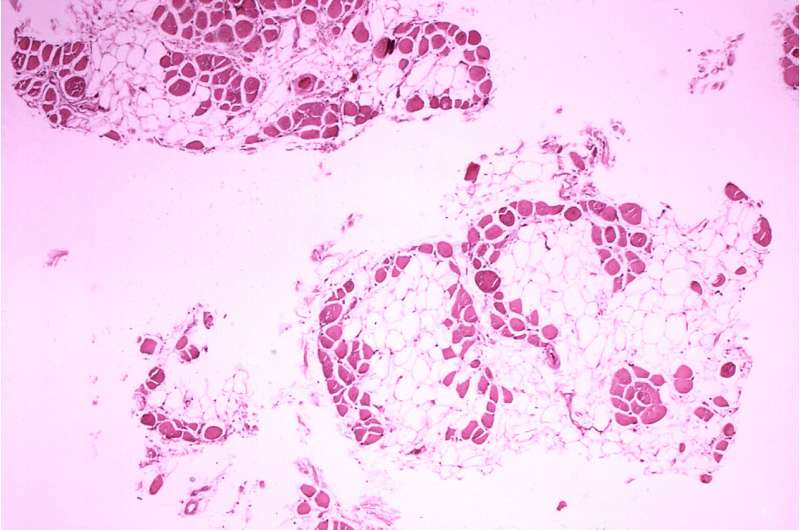
Francesco Muntoni, MD, from University College London, and coauthors, on behalf of the SKIP-NMD Study Group, conducted a two-part Phase 1/2 trial of long-term golodirsen treatment. Part 1 included a safety review. Part 2 was a 168-week evaluation of golodirsen 30 mg/kg. The primary endpoints were dystrophin protein expression and a 6-minute walk test. Golodirsen significantly increased dystrophin protein expression 16-fold and exon skipping 28.9-fold. After 3 years, the 6-minute walk test declined by only 99.0 meters for golodirsen-treated patients versus a decline of 181.4 meters for the control group, and loss of ambulation occurred in 2 of 25 golodirsen-treated versus 5 of 19 control patients.
“The results presented herein provide evidence for the biologic activity and long-term safety of golodirsen in a declining DMD population, supporting evaluation of golodirsen in an ongoing Phase 3 trial,” concluded the investigators.
Source: Read Full Article
