Gene therapy corrects mutation responsible for common heart condition, research shows
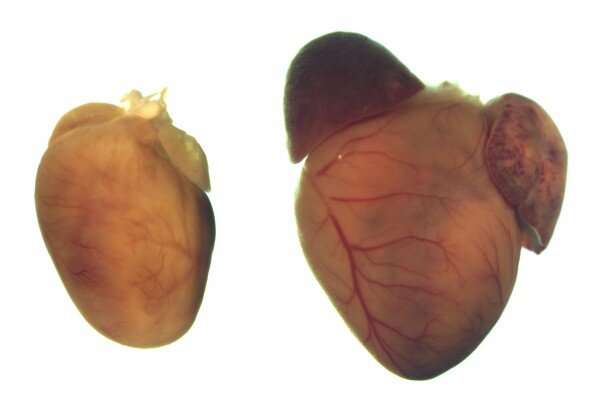
Using the CRISPR-Cas9 gene editing system, UT Southwestern researchers corrected mutations responsible for a common inherited heart condition called dilated cardiomyopathy (DCM) in human cells and a mouse model of the disease. Their findings, published in Science Translational Medicine, may one day provide hope to an estimated 1 in 250 people worldwide who suffer from this condition.
“All of the disease characteristics we see because of these mutations were reversed with CRISPR-Cas9 therapy. It’s fair to say the success of this approach completely exceeded our expectations,” said Eric Olson, Ph.D., Chair and Professor of Molecular Biology at UTSW, who co-led the study with colleagues Rhonda Bassel-Duby, Ph.D., Professor of Molecular Biology, and Takahiko Nishiyama, M.D., Ph.D., a postdoctoral fellow in the Olson lab.
DCM is caused by mutations in a gene known as RNA binding motif protein 20 (RBM20), which affects the production of hundreds of proteins in cardiac muscle cells responsible for the heart’s pumping action. This disease wreaks widespread havoc throughout the heart, gradually destroying its ability to contract and causing it to become extremely enlarged and fail over time. Treatment is limited to drugs, which can improve contractile function but don’t provide a permanent fix, or a heart transplant, which frequently isn’t an option due to a shortage of donor organs.
Seeking to attack the root cause of this disease, Drs. Olson, Bassel-Duby, Nishiyama, and their colleagues looked to CRISPR-Cas9, a popular tool for genetic research recognized with the Nobel Prize in Chemistry in 2020. Using this system, researchers can potentially correct disease-causing mutations in important genes.
Thus far, the Food and Drug Administration has approved a single clinical trial that uses this technology to try to treat sickle cell disease. However, Dr. Olson said CRISPR-Cas9 has huge potential to treat an untold number of other genetic diseases. Dr. Olson and colleagues have used CRISPR gene editing to develop a technique to halt progression of Duchenne muscular dystrophy in animal models.
To determine the feasibility of this approach for DCM, the research team used a virus to deliver CRISPR-Cas9 components to cardiac muscle cells derived from human cells carrying two different types of DCM-causing mutations. Scientists used this gene-editing technology to swap a single nucleotide, the basic unit of DNA, to correct one type of mutation. In another set of cells, researchers replaced a piece of DNA from mutated RBM20 with a healthy segment of this gene.
After CRISPR-Cas9 treatment, the mutant cells gradually lost characteristics inherent to DCM: The protein produced by RBM20 moved to its normal place in the nucleus, and the cells began making healthy proteins.
When the researchers delivered the CRISPR-Cas9 treatment to 1-week-old mice carrying one of these mutations, the animals never developed enlarged hearts and had normal life spans. Untreated mice had symptoms mirroring those of human DCM patients.
The scientists said that several challenges remain before this therapy can be used in DCM patients. Work is needed to ensure that the effects of CRISPR-Cas9 are permanent and precise, and that the smallest dose possible is delivered. Also to be determined is whether the treatment could be used in patients whose disease is more advanced. However, Dr. Olson said he’s optimistic that this system could be used to treat a variety of other familial diseases.
“The pace of this field is really breathtaking,” he said. “I expect that if this moves forward into patients, we’re not talking within decades—we’re talking within years.”
More information:
Takahiko Nishiyama et al, Precise genomic editing of pathogenic mutations in RBM20 rescues dilated cardiomyopathy, Science Translational Medicine (2022). DOI: 10.1126/scitranslmed.ade1633
Journal information:
Science Translational Medicine
Source: Read Full Article
