Human norovirus GII.4 exploits unexpected entry mechanism to cause gastroenteritis
Human noroviruses are the leading cause of acute gastroenteritis worldwide, a major global health problem for which there are no specific treatments or vaccines. Understanding the first phase of infection—the process the virus follows to invade cells—is a decisive step in the development of effective preventive and therapeutic strategies. A team led by researchers at Baylor College of Medicine is making strides in that direction.
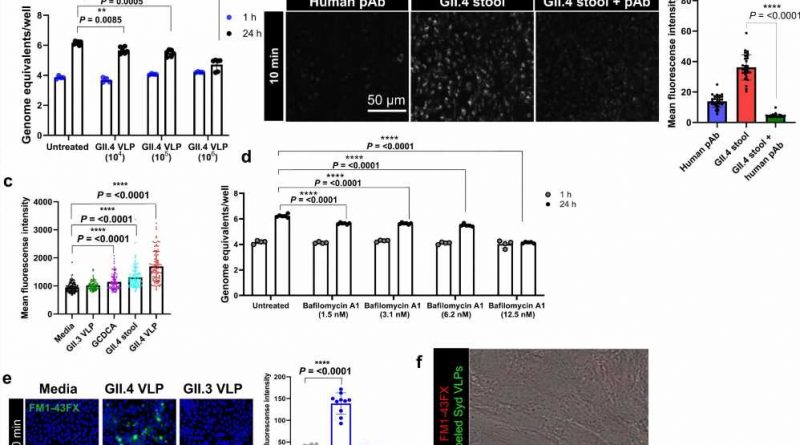
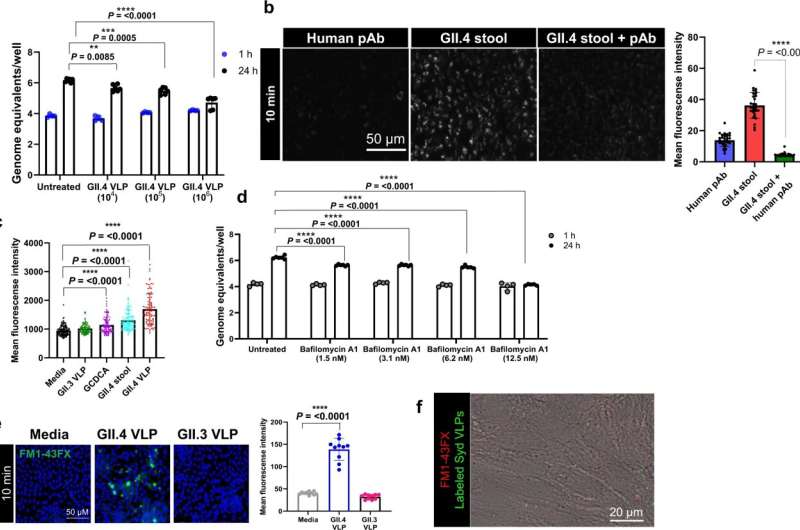
The researchers report in Nature Communications that the globally dominant human norovirus GII.4 strain invades gastrointestinal cells via an unexpected mechanism. The viral strategy involves interactions between specific components on both viral and human cell surface proteins and activates mechanisms that destabilize the cell membrane. The findings provide insight into the viral infection process, highlighting unique pathways and targets for developing effective therapeutics.
“We focused our study in the human norovirus pandemic strain GII.4, the one responsible for causing most cases of gastroenteritis around the world,” said first author Dr. B. Vijayalakshmi Ayyar, senior staff scientist of molecular virology and microbiology in the lab of Dr. Mary K. Estes at Baylor.
Ayyar, Estes and their colleagues worked with human intestinal enteroids, a laboratory model of the human gastrointestinal tract that recapitulates its cellular complexity, diversity and physiology. Human enteroids mimic strain-specific host-virus infection patterns, making them an ideal system to dissect human norovirus infection, identify strain-specific growth requirements and develop and test treatments and vaccines.
“We discovered that the binding of human norovirus GII.4 to enteroid cells wounds the cells’ membranes, which in turn triggers a membrane repair mechanism to the injury site, activating another cellular pathway known as the CLIC pathway,” Ayyar said. “We observed crosstalk between CLIC-mediated internalization of viral particles and host repair mechanisms. We propose that these pathways could be manipulated to interfere with viral entry in human intestinal cells.”
“To our knowledge, this is a previously uncharacterized complex entry process into human enteroids that combines several independent pathways. There may be more molecules involved in this entry pathway than what have been reported for other viruses,” said Estes, Distinguished Service Professor and Cullen Foundation Endowed Chair of molecular virology and microbiology at Baylor. Estes also is the corresponding author of the work.
“Other viruses use some components of the pathways mentioned in our paper, but this is the first time a virus has been shown to use all of them together. We now are interested in figuring out the role each of these molecules plays in this novel, interesting process, and whether it relates to the pandemic nature of GII.4.”
The team also discovered novel aspects of how the virus itself participates in the entry process.
“We know the virus structure is organized into two domains or parts, a shell domain and a protruding domain,” Ayyar said.
“Previously, we thought that all the interactions between the virus and cells involved only the protruding domain. In this work, we found that both the protruding and the shell domains are involved in the entry process. This suggests that virus interactions with cells cause changes in the viral structure that facilitate the cell entry. We also are interested in further exploring how these structural changes are induced and their precise role in the viral entry process.”
More information:
B. Vijayalakshmi Ayyar et al, CLIC and membrane wound repair pathways enable pandemic norovirus entry and infection, Nature Communications (2023). DOI: 10.1038/s41467-023-36398-z
Journal information:
Nature Communications
Source: Read Full Article