Infrared spectroscopic profiling can pick up molecular traces of solid tumors in blood
Cancers can grow in numerous places within our bodies and present tremendous threat to our health. But if one could spot cancerous growth early on, the chances to win over it would be higher. Are there ways of achieving this? The Broadband Infrared Diagnostics (BIRD) research team from the Laser Physics department of the LMU Munich revealed that infrared spectroscopic profiling can be used to pick up molecular traces that solid tumors leaves in our blood stream.
Detecting early and less aggressive cancerous lesions is paramount for having valid medical-treatment options. Besides radiographic tools to visualize tumor tissues within our body, and beyond cutting out tissue biopsies from inner organs for inspection under the microscope, modern diagnostic approaches often focus on non-invasive cancer detection: They analyze bodily fluids and try to capture macroscopically “invisible” molecular changes caused by cancer. In fact, tumors disseminate many aberrant metabolic products and signaling molecules into their surroundings. Likewise, tumors also characteristically interact with adjacent normal cells of a tissue, and later on with our immune cells and blood vessels. These interactions substantially affect the type and amount of many molecules that end up circulating in our bloodstream, even at a time when a tumor is still confined to an organ and not yet metastasizing. However, unambiguously identifying molecules that signify cancer—the holy grail for medical diagnostics and pharma—remains a challenge.
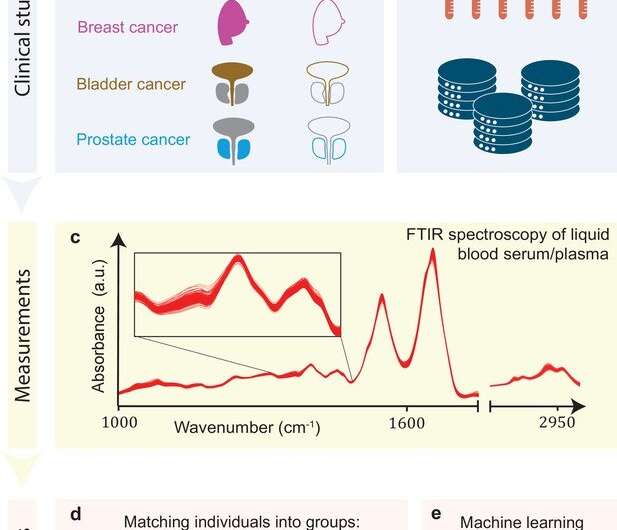
Substantial efforts have been made by the attoworld research team at the LMU Munich in paving new technological avenues for the most reliable and sensitive detection of molecules in complex liquid matrices. In this context, the BIRD research team just published a paper in eLife where they flowed a tiny volume of a blood sample through a cuvette, shined infrared light through it, and quantified the complex changes in the light-wave patterns coming from the blood sample as a function of the identity and number of the hundreds of thousands of different molecules dissolved therein. Extracting information by using machine learning algorithms allows determining a signature that is so highly characteristic of an individual’s blood sample that the signature can be referred to as a “molecular fingerprint.” The BIRD team’s earlier work published in Nature Communications showed that such infrared molecular fingerprints were highly reproducible over repeated blood draws from an individual.
The size of the problem in tracing disease like cancer by infrared molecular fingerprinting now becomes immediately obvious: One has to move to the population level. Thus scientists had to analyze these fingerprints of almost two thousand individuals to be in the position to extract the difference between the average healthy fingerprint and the average disease fingerprint. How does it work in real life? In collaboration with medical doctors from LMU clinics, the BIRD team set up a matched case-control clinical study and performed comparative infrared molecular fingerprinting on samples from patients with independently diagnosed lung, prostate, breast, or bladder carcinomas. And indeed, infrared fingerprinting of blood was surprisingly robust and correctly detected the cancer state. Excitingly, infrared fingerprints could be utilized, not only for detecting cancer but also for distinguishing between different cancer types, indicating that each had triggered specific molecular alterations.
Source: Read Full Article
