Inhibiting the biological crosstalk of autophagy and mitochondrial function underlying pancreatic ductal adenocarcinoma
Pancreatic ductal adenocarcinoma (PDAC) cells maintain a high level of autophagy or degradation, allowing them to thrive in severely limiting microenvironments. However, the process via which autophagy promotes pancreatic cancer cell growth and survival have yet to be understood.
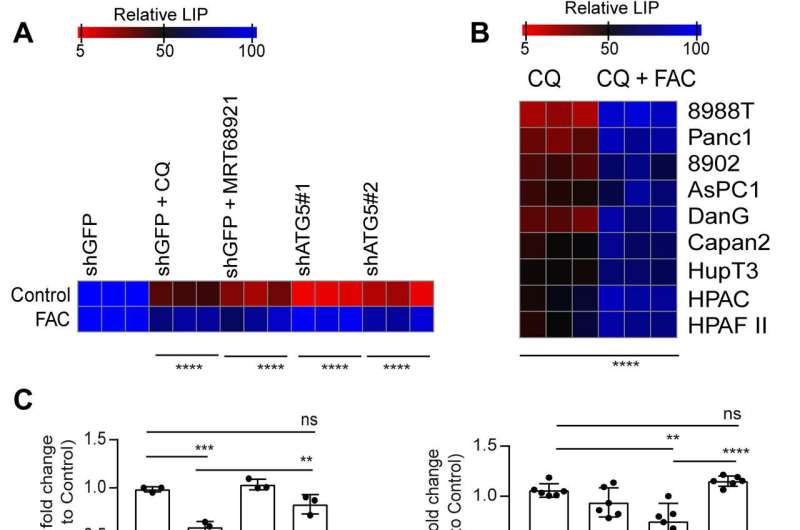
In a new report published in Science Advances, Subhadip Mukhopadhyay and a research team in radiation oncology and genome stability at the NYU and Harvard, showed how the inhibition of autophagy altered the mitochondrial function in PDAC patients. They experimentally attenuated the succinate dehydrogenase complex iron sulfur subunit in mitochondria to limit the availability of the labile iron pool.
The cancer cell lines used autophagy to sustain iron homeostasis in contrast to other tumor types that relied on macropinocytosis. The biologists noted how cancer-associated fibroblasts provided bioavailable iron to pancreatic cancer cells to resist the ablation of autophagy. Mukhopadhyay and colleagues administered a low-iron diet in a mouse model to hinder this crosstalk in the form of autophagy inhibition therapy. The outcomes emphasized a critical link between autophagy, iron metabolism and mitochondrial function to drive the progression of pancreatic ductal adenocarcinoma.
Iron homeostasis in pancreatic ductal adenocarcinoma (PDAC)
Pancreatic ductal adenocarcinoma represents more than 95% of pancreatic cancers that are highly resistant to therapy with a low 5-year survival rate, where the number of cancer-related deaths accounted to the second highest rate in the United States. These tumors are defined by a harsh, hypoperfused and hypoxic microenvironment with altered availability of nutrients. The cancer cells can survive hostile environments by reprogramming their metabolic needs to rely on nutrient scavenging mechanisms such as macroautophagy and macropinocytosis to survive and grow. Previous work had highlighted the role of ferritinophagy—a selective form of autophagy—and its role in iron homeostasis maintenance.
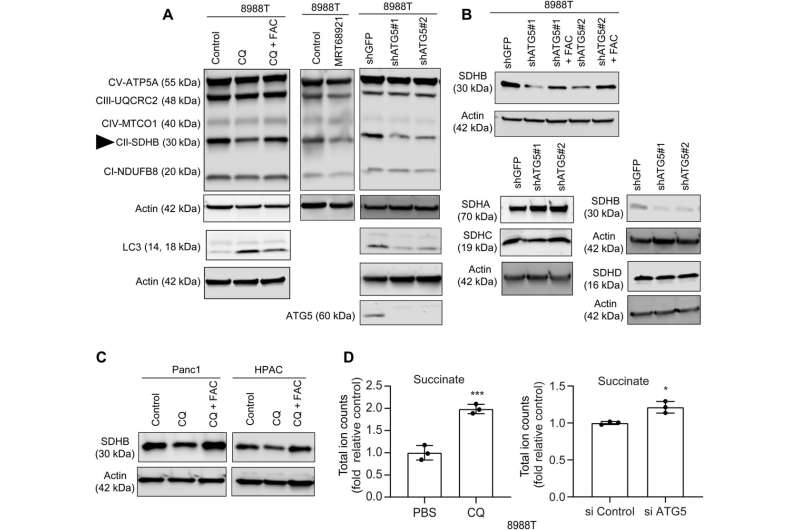
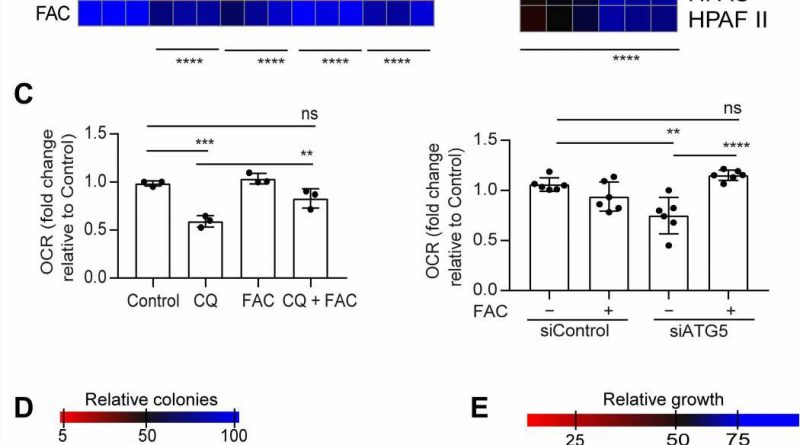
In this work, Mukhopadhyay and the team showed how the inhibition of autophagy reduced the assembly of iron-sulfur clusters to stabilize a variety of proteins, including those involved in the electron transfer chain, such as succinate dehydrogenase complex iron sulfur subunit B (SDHB). The reduction of this molecule in pancreatic cancer cells during autophagy led to a decrease in mitochondria respiration and reorganization of the cristae microarchitecture. The supplementation of iron or the expression of ectopic SDHB revived the growth and improved the mitochondrial defects.
Limiting the bioavailable iron in pancreatic ductile cancer cells to inhibit autophagy
Previously, Mukhopadhyay and colleagues showed how autophagy inhibition in pancreatic cancer cell lines led to a decrease in the mitochondrial oxygen consumption rate. However, they could not determine the mechanism of this impairment. Prior research had shown amino acid pools to remain unaltered upon autophagy inhibition, except for a decrease in cysteine levels.
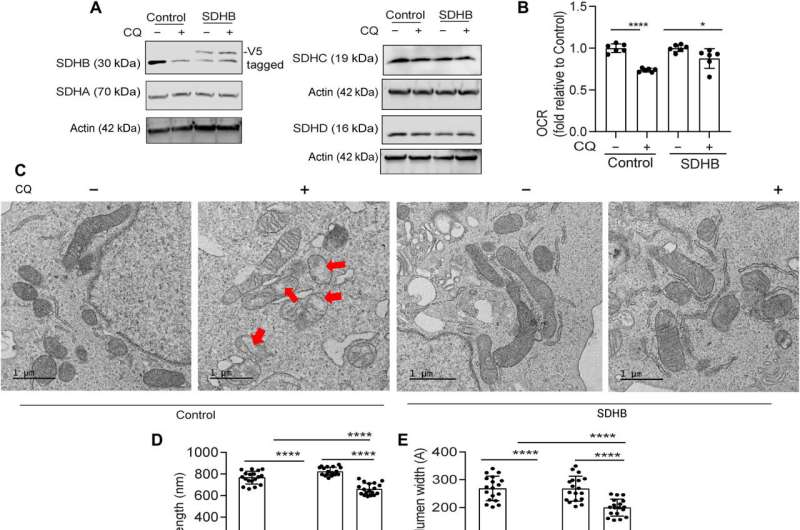
However, supplementing cysteine to pancreatic cancer cells did not alter the defective rate of oxygen consumption, indicating a pathway that directly affected the mitochondria. While iron-sulfur cluster proteins drove mitochondrial function, a drop in the labile iron pool after autophagy inhibition decreased mitochondrial respiration, which the team restored via ferric ammonium citrate supplementation. The inhibition of autophagy further led to decreased levels of the succinate dehydrogenase complex iron sulfur subunit B (SDHB) protein, highlighting a functional loss of the mitochondrial protein.
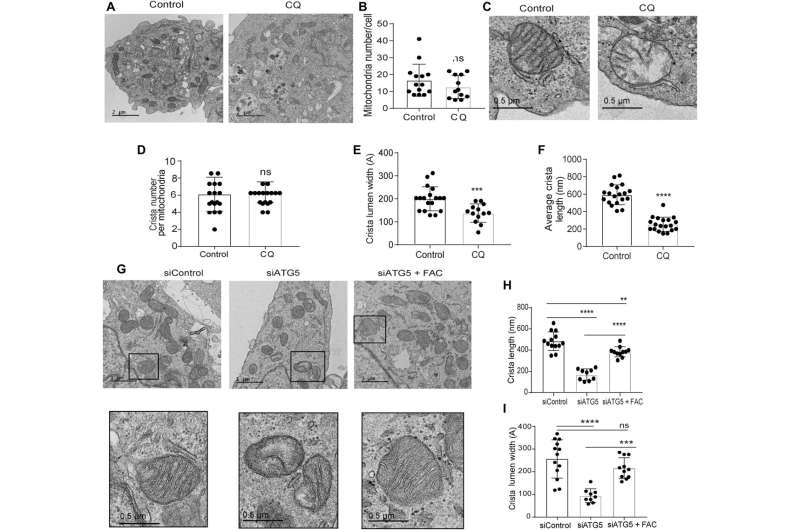
Defects in the mitochondrial architecture are related to autophagy inhibition
The research team used transmission electron microscopy to reveal the mitochondrial architecture of autophagy-deficient pancreatic cancer cells; enabled by administering chloroquine. They noted a significant reduction in mitochondrial cristae lumen with large vacant spaces post-treatment to indicate functional defects.
The team showed how the ectopic expression of the SDHB protein could restore the cristae microarchitecture. In fact, overexpression of SDHB rescued the proliferation and oxygen consumption rate after autophagy inhibition. They studied the mechanism via which these components played a significant role during the biogenesis of iron and sulfur proteins, after the loss of autophagy in pancreatic cancer cells.
Investigating the crosstalk between cancer-associated cell co-cultures with drug moieties
While autophagy can regulate the availability of iron in pancreatic cancer cells, the patient response to hydroxychloroquine monotherapy was not clinically sufficient to attenuate the disease. To examine the impact of the tumor microenvironment to this resistance, the researchers studied the effects of compensating the labile iron pool by co-culturing pancreatic cancer cells with cancer-associated fibroblasts to recreate a microphysiological tumor environment.
The researchers studied the crosstalk between cells to understand cellular regulation of the labile iron pool and examined the expression of key iron transporters. They noted an increase in the exporter ferroprotein in fibroblasts cultured alongside autophagy-inhibited pancreatic cancer cells. When they administered Tocilizumab, they restored the antiproliferative effects of autophagy inhibition on the cancer cell line in the co-culture system.
Treating pancreatic ductal adenocarcinoma (PDAC)
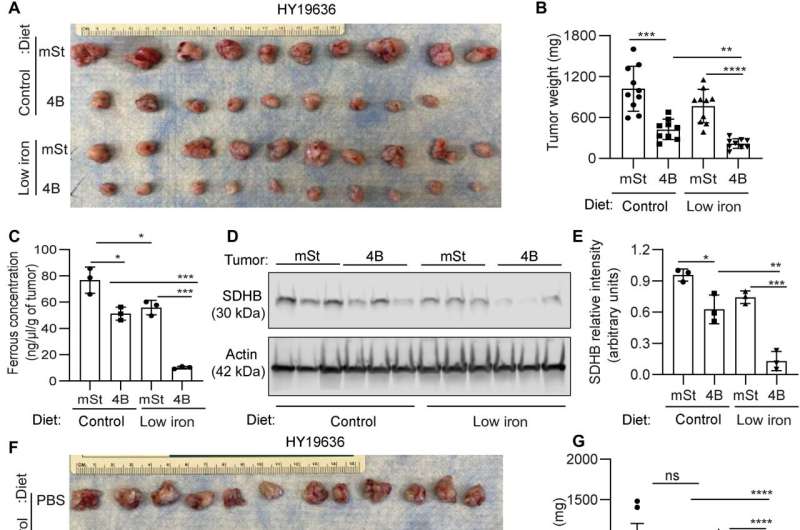
Mukhopadhyay and colleagues explored the methods to treat the cancer and explored if autophagy inhibition could facilitate iron restriction to inhibit pancreatic tumor growth. They decreased the bioavailable iron to impair the crosstalk between pancreatic duct adenocarcinoma cells and cancer-associated fibroblasts. They accomplished this by systematically reducing iron levels in mice by exposing them to a low-iron diet for two weeks before transplanting cancer cells with a doxycycline-inducible dominant vector and a control vector in mouse hosts. While the iron level in the diet did not affect the level of hemoglobin, autophagy inhibition significantly decreased tumor growth. The low-iron diet further reduced tumor growth to suggest that iron deprivation sensitized tumor cells to undergo autophagy inhibition.
The combination of an iron-limiting diet and autophagy decreased the bioavailable ferrous iron concentration in tumors, while decreasing the succinate dehydrogenase complex iron sulfur subunit B expression. When the scientists administered chloroquine alone, it failed to reduce tumor growth in mice, although combined chloroquine and an iron-restricted diet significantly reduced tumor growth. The combined data represented a low-iron diet that worked together with autophagy inhibition to critically disrupt iron homeostasis in pancreatic ductal adenocarcinoma tumors.
Outlook
In this way, Subhadip Mukhopadhyay and colleagues identified how pancreatic duct adenocarcinoma tumors relied on autophagy to grow and evolve with time during disease progression. Autophagy inhibition decreased the labile iron pool and the synthesis of iron-sulfur clusters. They supplemented the cultures with iron and promoted the ectopic expression of both iron-sulfur clusters and succinate dehydrogenase complex iron sulfur subunit B (SDHB) protein to restore mitochondria function and cell growth. Autophagy was essential to maintain iron homeostasis, and support mitochondrial function, required for both growth and proliferation, in pancreatic cancer cells.
The researchers dissected multiple metabolic cross-talks occurring between the tumor and stroma, and explored the disruption of iron metabolism in the pancreatic duct adenocarcinoma cells and its impact on the complex tumor microenvironment. These mechanistic findings will assist in the development of new design approaches to target the process of iron metabolism in pancreatic duct adenocarcinoma cells.
More information:
Subhadip Mukhopadhyay et al, Autophagy supports mitochondrial metabolism through the regulation of iron homeostasis in pancreatic cancer, Science Advances (2023). DOI: 10.1126/sciadv.adf9284
Cristovão M. Sousa et al, Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion, Nature (2016). DOI: 10.1038/nature19084
Journal information:
Science Advances
,
Nature
Source: Read Full Article