Maintenance of self-renewal and proliferation of hippocampal neural stem cells during postnatal development
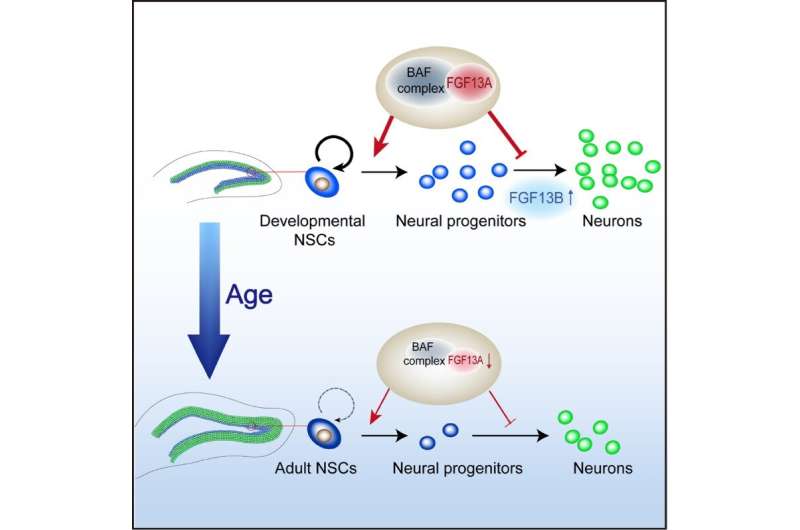
In a study published in Cell Reports, Dr. Zhou Jiawei’s Lab at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology (CEBSIT) of the Chinese Academy of Sciences, and Dr. Hu Gang’s group at the Nanjing Medical University, demonstrated that FGF13 gene acts as an intrinsic regulator in the maintenance of the self-renewal and proliferation of neural stem cells (NSCs) in the hippocampus during the postnatal development.
The development and plasticity of the hippocampal dentate gyrus (DG) are crucial for behaviors, such as learning and memory. In the mouse brain, most of granule cells in the DG are generated during early postnatal stages. The neurogenic capacity of NSCs in the DG drops sharply during the first two postnatal weeks and then gradually declines with age in adulthood.
FGF13 belongs to a subgroup of non-secretory fibroblast growth factor (FGFs) family. Human genetic studies have revealed that Fgf13 is a candidate gene of several X-linked brain disorders including mental retardation and epilepsy with febrile seizures plus, indicating the important roles of FGF13 in brain development. A previous study by CEBSIT shows that FGF13 takes part in regulating polarity/migration of immature neurons in the forebrain during embryonic stage.
In this study, the researchers found that expression of isoform A of FGF13 was decreased in NSCs with age-related decline of DG neurogenesis, and deficiency of FGF13 in NSCs caused remarkable impairment of postnatal neurogenesis in the DG and hippocampus-related learning and memory behaviors of mice.
Mechanistically, FGF13A isoform is essential for maintaining the capacity of self-renewal of NSCs by modifying chromatin structure and regulating the genes involved in neuron differentiation. The yeast two-hybrid assay suggested that FGF13A interacted with ARID1B, a crucial component in the Brahma-associated factor (BAF) chromatin remodeling complex thereby regulating the development of NSCs.
Additionally, the results of assay for transposase accessible chromatin with high throughput sequencing (ATAC-seq) suggested that FGF13 is required for the maintenance of close chromatin state of neuronal differentiation-related genes in order to maintain the self-renewal and proliferative state of NSCs. Thus, the normal development of hippocampus requires proper spatiotemporal coordination of FGF13 isoform expression.
Source: Read Full Article
