New process produces purer, safer pancreas stem cells for potential transplant
A University of Alberta team has developed a new step to improve the process for creating insulin-producing pancreatic cells from a patient’s own stem cells, bringing the prospect of injection-free treatment closer for people with diabetes.
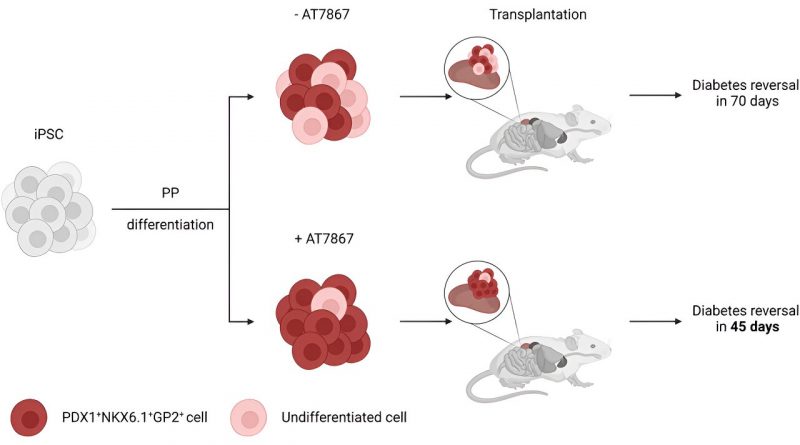
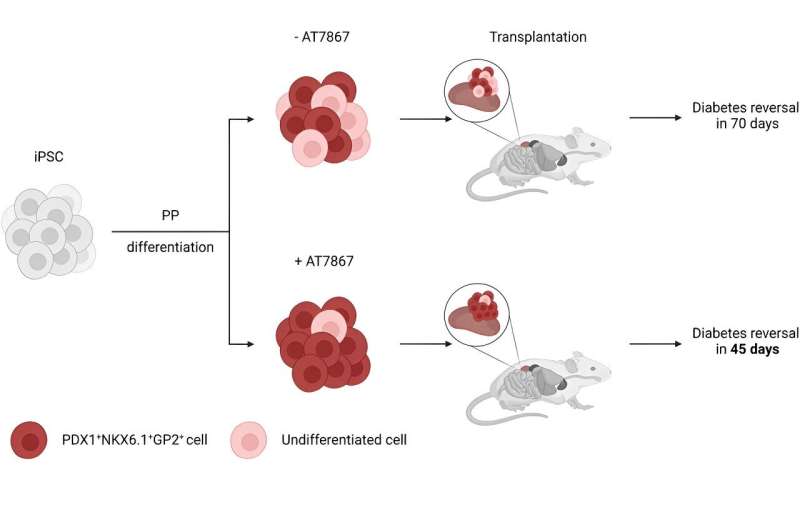
In research published this month, the team treated pancreatic progenitor cells with an anti-tumor drug known as AKT/P70 inhibitor AT7867. They report the method produced the desired cells at a rate of 90 percent, compared with previous methods that produced just 60 percent target cells. The new cells were less likely to produce unwanted cysts and led to insulin injection-free glucose control in half the time when transplanted into mice.
The researchers suggest this safer and more reliable way to grow insulin-producing cells from a patient’s own blood could eventually allow transplants without the need for anti-rejection drugs.
“We want to replace the need for insulin injections with cells that produce the same hormone—insulin—that will regulate blood sugar in a perfect manner,” says James Shapiro, Canada Research Chair in Transplant Surgery and Regenerative Medicine, professor of medicine and surgical oncology, and head of the Edmonton Protocol, which has allowed 750 transplantations of donated islet cells since it was first developed 21 years ago.
Recipients of donated cells must take anti-rejection drugs for life, and the therapy is limited by the small number of donated organs available.
“We need a stem cell solution that provides a potentially limitless source of cells,” says Shapiro, “and we need a way to make those cells so that they can’t be seen and recognized as foreign by the body’s immune system.”
The researchers take stem cells from a single patient’s blood and chemically wind them back in time, then forward again in a process called “directed differentiation,” to eventually become insulin-producing cells.
“The cells used for this research were pancreatic progenitors, cells that can be further differentiated in the lab to generate cells that release insulin,” says Nerea Cuesta-Gomez, a postdoctoral fellow with the Clinical Islet Transplant Program, which Shapiro directs. “Throughout those next steps of differentiation, we believe that we will be able to eliminate that five to 10 percent of cells that did not result in pancreatic cells.”
Regardless of whether the cells belonged to a patient with Type 1 or Type 2 diabetes, or someone who has surgical diabetes due to removal of the pancreas, all the cells were able to reverse diabetes, Cuesta-Gomez notes.
Shapiro cautions that further safety and efficacy studies will need to be carried out on the method before transplantation of stem-cell-derived islet cells is ready for human trials, but he is excited by the progress.
“What we’re trying to do here is peer over the horizon and try to imagine what diabetes care is going to look like 15, 20, 30 years from now,” he says. “I don’t think people will be injecting insulin anymore. I don’t think they’ll be wearing pumps and sensors.”
“If I reflect on the cumulative diabetes research that I’ve been involved with over the last 40 years of being involved with diabetes research since I was a medical student, this is by far the most costly, the most ambitious, but also the most exciting project I’ve been involved in.”
The research is published in the journal Stem Cell Reports.
More information:
Nerea Cuesta-Gomez et al, AT7867 promotes pancreatic progenitor differentiation of human iPSCs, Stem Cell Reports (2023). DOI: 10.1016/j.stemcr.2023.10.005
Journal information:
Stem Cell Reports
Source: Read Full Article