Plant-based virus-like particle vaccine induces durable immune response against SARS-CoV-2 variants
Scientists from Germany and Belgium have recently described the immunogenicity of a plant-based virus-like particle vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The vaccine induces robust antibody and T cell responses for at least 6 months. Moreover, the vaccine demonstrates potent neutralizing efficacy against the B.1.1.7, B.1.351, and P1 variants of SARS-CoV-2. The study is currently available on the medRxiv* preprint server.
.jpg)
Background
The COVID-19 outbreak is the biggest pandemic in modern history, with more than 203 million affected people and 4.31 million deaths. Because of the limited availability of specific treatments, management of the COVID-19 pandemic mostly depends on mass vaccination together with non-pharmacological control measures.
The anti-pathogen efficacy of a vaccine primarily depends on its ability to induce neutralizing antibodies. Moreover, the ability to induce cross-reactive antibodies is another vital measure to protect against emerging viral variants. According to available evidence, most of the COVID-19 vaccines are capable of inducing neutralizing antibody titers for at least 8 months.
In the current study, the scientists have described the robustness and durability of immune responses induced by an AS03-adjuvanted, plant-based virus-like particle vaccine containing full-length spike protein of wildtype SARS-CoV-2 as an immunogen.
Study design
The study was conducted on 20 healthy adults who received two doses of 3.75 µg vaccine at an interval of 21 days. The blood samples were collected from the participants 21 days after each immunization and 6 months after the second immunization.
Antibody-mediated immune responses
Immunoglobulin G (IgG)-specific anti-spike binding antibodies were detected in all participants 21 days after the second vaccine dose. The antibodies remained detectable for at least 6 months after the final vaccination. The levels of spike-binding antibodies detected at day 21 post first vaccination were comparable to that observed in COVID-19 recovered patients; however, the levels were significantly lower than that detected at day 21 post-second vaccination.
The neutralizing antibody levels peaked at day 21 after the second vaccination, followed by a gradual decline. However, the levels detected after 6 months of second vaccination were comparable to that in convalescent plasma.
According to the exponential-decay model, the estimated half-life of binding and neutralizing antibodies was around 55 days.
Cell-mediated immune response
Interferon γ (IFN-γ)-secreting type 1 T helper cell response was detected in all participants at day 21 post final vaccination. The response remained detectable even after 6 months. A similar trend was also observed for interleukin-4 (IL-4)-secreting type 2 T helper cell response.
The intensity of both IFN-γ and IL-4 responses was highest at day 21 post final vaccination, followed by a gradual decline. Despite the reduction in intensity, the presence of T cell response in almost all participants after 6 months of final vaccination highlights the vaccine's effectiveness in inducing a durable cellular immune response.
Antibody cross-reactivity
Serum samples obtained from vaccinated individuals and COVID-19 recovered patients showed high reactivity to SARS-CoV-2 spike protein. However, the magnitude of reactivity was higher for vaccinated individuals. In addition, significantly higher levels of cross-reactive antibodies against the SARS-CoV spike protein were observed in vaccinated sera compared to that in convalescent sera. Neither significant cross-reactivity to the spike proteins of Middle East respiratory syndrome coronavirus and common cold coronavirus was observed in both vaccinated and convalescent sera.
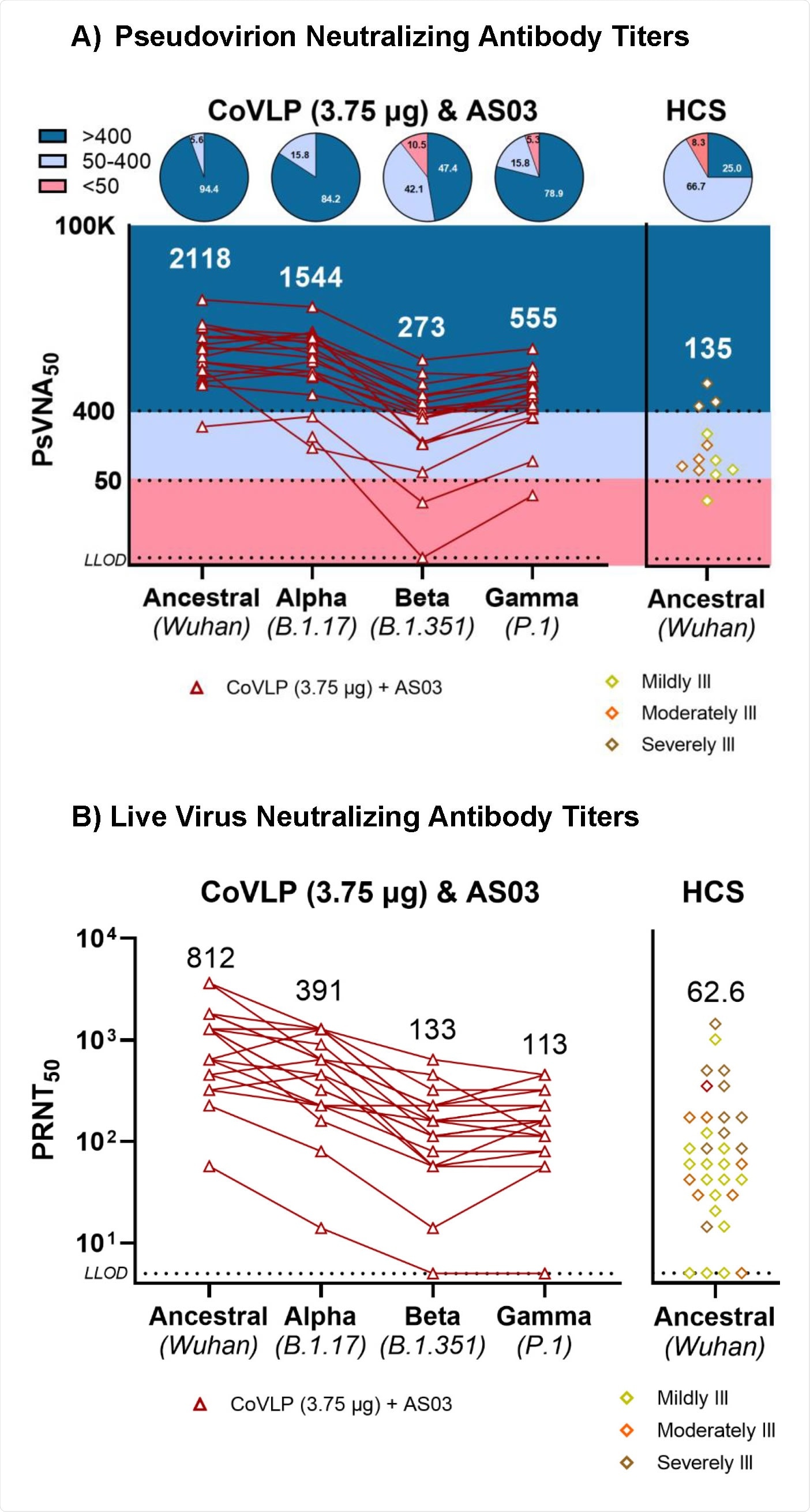
Regarding SARS-CoV-2 variants of concern (VOCs), all participants exhibited antibody-mediated neutralization of the B.1.1.7, B.1.351, and P1 variants at day 21 post final vaccination. Overall, the highest neutralizing antibody titer was observed against the B.1.1.7 variant, and the lowest titer was observed against the B.1.351 variant.
Taken together, the study highlights the potency of a plant-based virus-like particle vaccine in inducing robust humoral and cellular immune responses after 21 days of final immunization. Although lower in magnitude, the responses remain detectable even after 6 months. Importantly, at day 21 post final vaccination, almost all vaccine recipients have exhibited detectable neutralizing antibodies against some of the SARS-CoV-2 VOCs.
The vaccine is currently under investigation in a large phase 3 clinical trial involving many countries with the active circulation of multiple VOCs.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Gobeil P. 2021. Durability and Cross-Reactivity of Immune Responses Induced by an AS03 Adjuvanted Plant-Based Recombinant Virus-Like Particle Vaccine for COVID-19, medRxiv, https://doi.org/10.1101/2021.08.04.21261507, https://www.medrxiv.org/content/10.1101/2021.08.04.21261507v1
Posted in: Medical Research News | Disease/Infection News
Tags: Antibodies, Antibody, Assay, Blood, Cell, Clinical Trial, Cold, Common Cold, Convalescent Plasma, Coronavirus, Coronavirus Disease COVID-19, Efficacy, Immune Response, Immunization, Immunoglobulin, Pandemic, Pathogen, Protein, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Vaccine, Virus

Written by
Dr. Sanchari Sinha Dutta
Dr. Sanchari Sinha Dutta is a science communicator who believes in spreading the power of science in every corner of the world. She has a Bachelor of Science (B.Sc.) degree and a Master's of Science (M.Sc.) in biology and human physiology. Following her Master's degree, Sanchari went on to study a Ph.D. in human physiology. She has authored more than 10 original research articles, all of which have been published in world renowned international journals.
Source: Read Full Article
