Research lays groundwork for promising new gene therapy approach for genetic heart disease: Clinical trials imminent
Researchers at the Hubrecht Institute have laid the foundation for the development of a gene therapy for the genetic heart disease arrhythmogenic cardiomyopathy (ACM). Their approach, based on replacement of the PKP2 gene, led to significant structural and functional improvements in laboratory models of the disease.
The study by the group of Eva van Rooij was published on 7 December 2023 in Nature Cardiovascular Research. Multiple clinical trials will start in 2024 in the United States to explore the clinical potential of this approach in ACM patients with PKP2 mutations.
ACM is a genetic heart disease that affects 1 in 2,000 to 1 in 5,000 people worldwide. It is characterized by arrhythmias and can lead to sudden cardiac arrest. Current treatment of the disease usually consists of antiarrhythmic drugs and implantable cardioverter-defibrillators (ICDs), which are focused solely on treating the symptoms rather than targeting the root of the problem.
The disease is progressive, with an increasing part of the heart muscle being replaced by fat tissue and heart function deteriorating over time. This can eventually lead to heart failure. In severe cases, heart transplantation can be performed as a last resort, but this is complicated by long waiting lists as a result of the limited availability of suitable donor hearts. There is, therefore, an urgent need for effective treatments that target the cause of ACM.
Disease of the desmosome
The mutations at the basis of ACM often occur in genes related to the desmosomes. These protein structures form the connections between adjacent heart muscle cells. Not only do they provide a structural link, but they also ensure that the heart muscle cells contract synchronously, allowing the heart to pump blood in a coordinated manner.
The most frequently affected gene in ACM is PKP2, which encodes the plakophilin-2 protein, an essential part of the desmosomes. The first author of the study, Eirini Kyriakopoulou, explains the effect of PKP2 mutations: “Patients with mutations in this gene often have lower levels of the plakophilin-2 protein in their heart muscle cells.”
“The result is that the desmosomes, which are normally built up by meticulously binding all proteins together, begin to fall apart and are broken down by the cell. This weakens the connections between the heart muscle cells, which makes it difficult for them to work together in synchrony, leading to the development of arrhythmias.”
Gene therapy
With the molecular cause of ACM in mind, the researchers set out to develop a therapeutic approach that would target this cause and not just the symptoms.
“For many patients with PKP2 mutations, the root of the problem is insufficient levels of plakophilin-2. Therefore, we explored the potential of gene therapy in ACM. We hypothesized that by introducing the healthy PKP2 gene into affected heart muscle cells, we might be able to restore plakophilin-2 levels to normal, thereby reinforcing the desmosomes and reducing the occurrence of arrhythmias in these patients,” says Kyriakopoulou.
Improved heart function in the laboratory
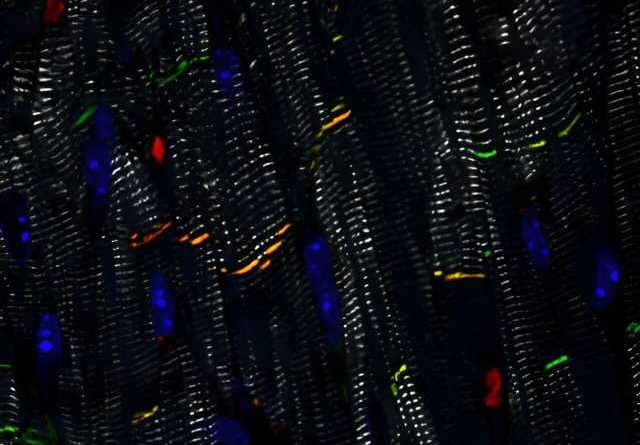
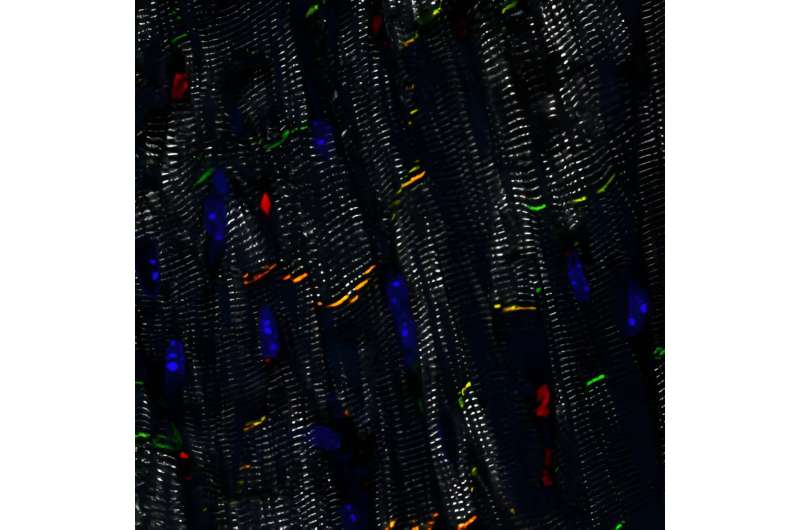
Using several laboratory models of ACM, Kyriakopoulou and her colleagues demonstrated both the feasibility and the efficacy of delivering the healthy PKP2 gene to diseased heart muscle cells. “We showed that plakophilin-2 levels were restored after delivery of the gene to human heart muscle cells grown from stem cells. It also improved their sodium conduction, which is important for their ability to contract.”
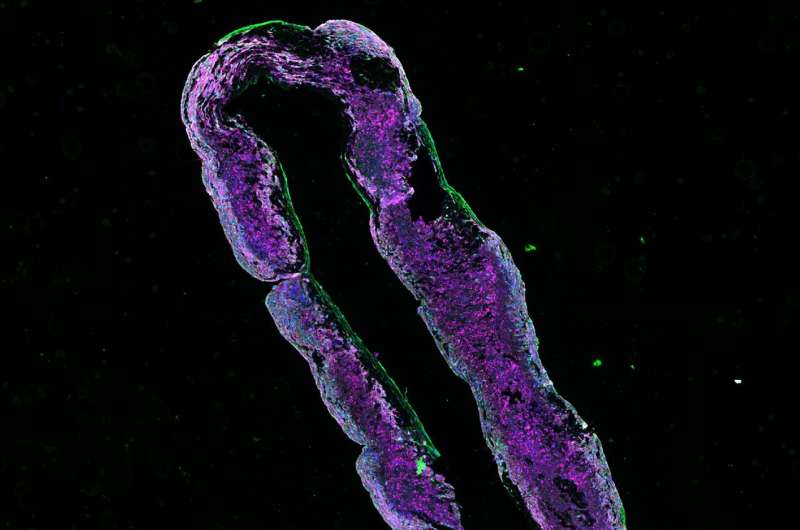
“We then confirmed this improvement of contractility in engineered human heart muscles, which are ring-shaped structures that we can grow in the lab. Heart muscles with a PKP2 mutation contracted better after receiving the healthy PKP2 gene. Finally, we wanted to test this strategy in vivo, so we applied PKP2 gene replacement in our mouse model of ACM. This led to recovery of their desmosomes and heart function,” Kyriakopoulou explains.
From the lab to the clinic
Following the promising laboratory results, the next step is to investigate the clinical potential of this gene therapy approach in ACM patients with PKP2 mutations. “Three companies in the United States have announced that they will start clinical trials next year to test the therapeutic effect of this approach in patients, which is of course great news,” says Kyriakopoulou. The researchers at the Hubrecht Institute hypothesize that gene replacement therapy would be most useful in the early stages of the disease.
Kyriakopoulou says, “Once the disease has progressed so much that parts of the heart muscle have already been replaced by fat tissue, it is uncertain whether this approach would reverse already existing damage. Instead, we believe that it might be possible to prevent progression of early-stage disease to more severe stages.”
Although the pre-clinical results and upcoming trials hold great promise, Kyriakopoulou emphasizes that the commercial availability of this approach could still take several years. “Apart from the evident need to confirm its efficacy in patients, it is also crucial to address and eliminate any safety concerns before considering its clinical application. Nevertheless, our work provides an important basis to build on.”
More information:
Eirini Kyriakopoulou et al, Therapeutic efficacy of AAV-mediated restoration of PKP2 in arrhythmogenic cardiomyopathy, Nature Cardiovascular Research (2023). DOI: 10.1038/s44161-023-00378-9
Journal information:
Nature Cardiovascular Research
Source: Read Full Article