Researchers find cold-activated brown fat asks for more sugar via signaling RNAkines
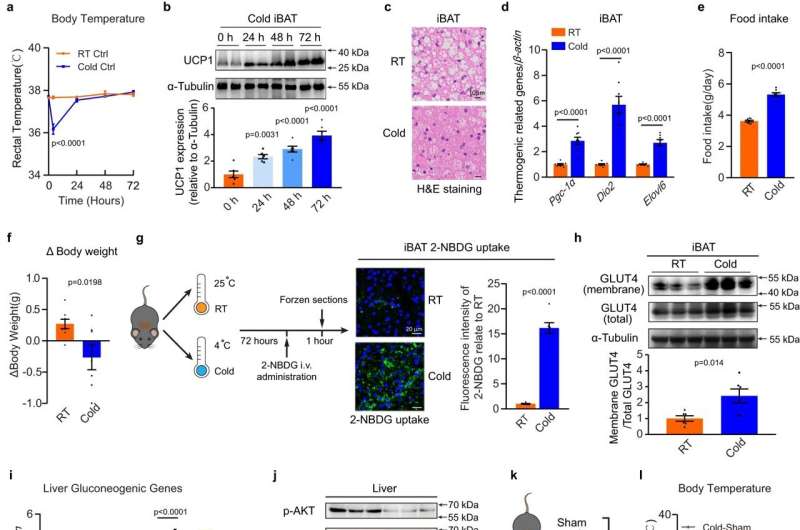
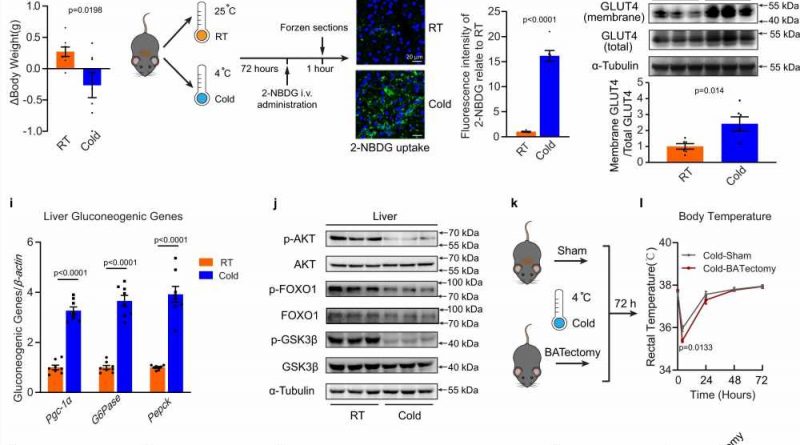
Brown adipose tissue (BAT) is the primary site of non-shivering thermogenesis (NST), which protects mammals from hypothermia. NST is significantly increased in response to cold exposure and dissipating energy to produce heat. To replenish intracellular energy stores, cold-activated BAT requires sharply increased uptake of glucose and fatty acids as fuel supplies to sustain high levels of mitochondria respiration.
As landmark papers have demonstrated that active BAT present in adult humans when exposed to cold, studies have been specifically designed to investigate the role of activated BAT. Notably, cold exposure did not affect whole-body glucose metabolism in men who did not have detectable BAT, indicating a physiologically significant role of activated BAT in regulating whole-body glucose homeostasis.
In addition to its role in thermogenesis, BAT also functions as an endocrine organ, which secretes peptidic and nonpeptidic molecules, such as bioactive lipids and miRNAs. But what is the endocrine function of BAT in controlling glucose homeostasis under the cold challenge? Nanjing University Lab and Cambridge Metabolic Research Lab physiological researchers are asking the question to understand the specific role of “Batokines.”
In a new article published in Nature Communications, the researchers report the finding that in male mice, cold exposure facilitates the EV secretion and selective packaging of miR-378a-3p—into EVs and delivery into the liver. BAT-secreted miR-378a-3p enhances hepatic gluconeogenesis by targeting p110α. miR-378 knockout mice display reduced hepatic gluconeogenesis during cold exposure, while restoration of the miRNA in BAT partially rescues the phenotype.
Cold and fasting are the two fundamental challenges in mammals during evolution. They need to maintain a narrow and constant blood glucose range under these conditions, which requires a complex balance between glucose uptake and endogenous glucose production. In this study, the authors report that miR-378a-3p, contained in BAT-secreted extracellular vesicles target the liver to promote gluconeogenesis. This is a new adaptive mechanism to ensure glucose homeostasis in conditions of intense draining of glucose by activated BAT.
There are few studies on the role of BAT secretomes on hepatic gluconeogenesis. This study demonstrates that upon cold exposure, in addition to the classic adrenergic stimulation, RNAkine-mediated BAT-liver crosstalk also contributes to this physiological process to maintain whole-body glucose balance. It is a novel mechanism to understanding the RNAkines as stress-induced Batokines to coordinate systemic glucose homeostasis when facing cold challenge.
Cold stress promoted BAT-derived EVs secretion, and the miRNAs were selectively packaged into the BDEVs during cold exposure. These results further support that sequence motifs present in miRNAs could control their loading into EVs. The sequence motif which is present in miRNAs that tend to be localized in EVs, was also found in the mature sequence of miR-378a-3p.
Considering the biomedical implications of studies on BAT activity in relation to metabolic disorders, this study suggests that determining the pros and cons of identifying BAT as eliciting hepatic gluconeogenesis activation in the context obesity/diabetes would be of interest.
More information:
Jinhong Xu et al, Cold-activated brown fat-derived extracellular vesicle-miR-378a-3p stimulates hepatic gluconeogenesis in male mice, Nature Communications (2023). DOI: 10.1038/s41467-023-41160-6
Journal information:
Nature Communications
Source: Read Full Article