Researchers solve structure of immune-evading HIV protein complex
The HIV-1 virus can neutralize cellular defenses with its viral infectivity factor (Vif). OIST researchers Prof. Matthias Wolf and Dr. Takahide Kouno together with an international team of colleagues have now determined the atomic structure of the APOBEC3G-Vif complex using cryo-electron microscopy.
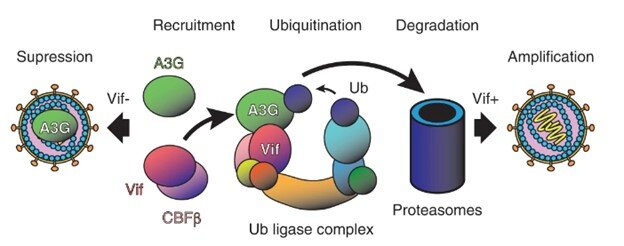
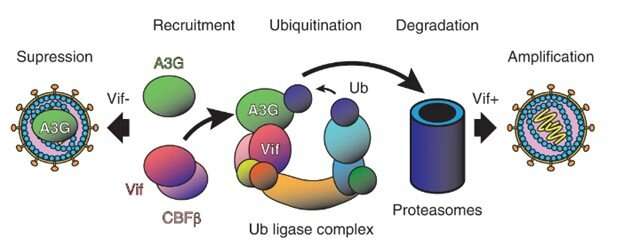
“APOBEC3G (A3G) is a key component of the human innate immune system to defend against invading viruses, getting a ride inside budding virions like in a Trojan horse so that it can modify and disable viral DNA after reverse transcription in infected cells,” explains Prof. Wolf, the senior author of the study and who leads the OIST Molecular Cryo-Electron Microscopy Unit.
“But HIV-1 has evolved a counteraction mechanism in the form of its Vif protein, which inhibits this process by binding to and degrading A3G, leading to successful amplification of infectious viral particles.”
A unique aspect of their study is the first demonstration that this complex formation is mediated by specific RNA sequences, even though single-stranded DNA is the substrate of degradation by A3G. Their protein construct was optimized for higher solubility and could replicate the degradation pathway by A3G ubiquitination (a process that involves the transfer of ubiquitin polyubiqitination to a target protein and is important in many physiological functions) in vitro.
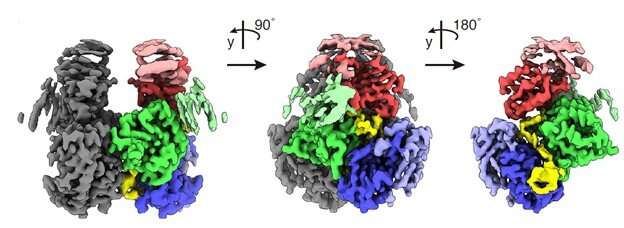
The work identified specific interactions between protein and the RNA ligand, which may be targeted with drugs for future antiviral therapies against HIV/AIDS that inhibit the A3G-Vif interaction.
The study was published in Nature Communications.
More information:
Takahide Kouno et al, Structural insights into RNA bridging between HIV-1 Vif and antiviral factor APOBEC3G, Nature Communications (2023). DOI: 10.1038/s41467-023-39796-5
Journal information:
Nature Communications
Source: Read Full Article