Scientists decipher the danger of gummy phlegm in severe COVID-19
Stanford University scientists have implicated a logjam of three long, stringy substances behind deadly thick sputum in COVID-19 patients who need a machine to help them breathe. One of these substances may prove especially amenable to treatment with a drug invented long ago for another purpose. It may also play a role in long COVID.
Their study was the first to analyze in depth the makeup, viscosity and immunological characteristics of sputum from the lungs of patients with severe cases of COVID-19, said Paul Bollyky, MD, Ph.D., an associate professor of infectious diseases and of microbiology and immunology.
Sputum, also known as phlegm, is the elephant in the room that is COVID-19.
“Thick, gummy respiratory secretions are at the heart of severe COVID-19,” Bollyky said. “But while tens of thousands of studies have analyzed COVID-19 patients’ blood samples, people haven’t looked much at seriously ill COVID-19 patients’ sputum samples—not least because they’re so hard to get.”
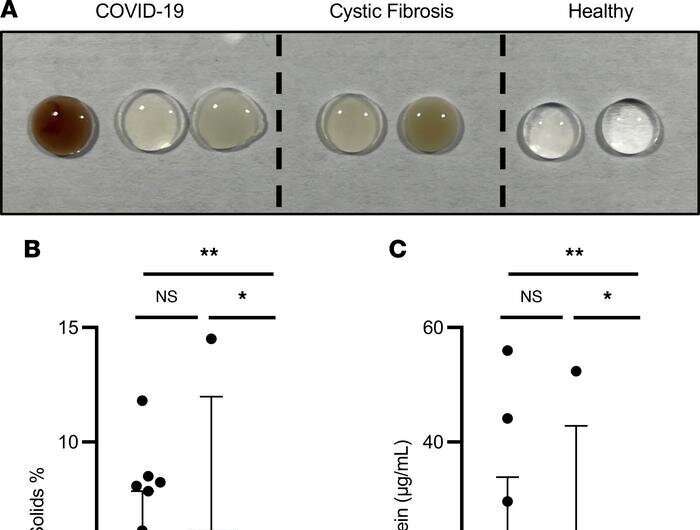
In the study, published online June 22 in JCI Insight, a team of pulmonologists, materials scientists and infectious disease specialists found three substances tangled up in the sputum of COVID-19 patients whose condition is severe enough that they need to be intubated and undergo mechanical ventilation. These tangles turn patients’ sputum into stiff stuff that’s tough to cough up, recalcitrant to oxygen exchange in the lungs and prone to inflammation—as well as consequent fluid buildup.
Bollyky shares senior authorship of the study with Carlos Milla, MD, professor of pediatric pulmonary medicine; Angela Rogers, MD, associate professor of pulmonary and critical care; Andrew Spakowitz, Ph.D., professor of chemical engineering and of materials science and engineering; and Sarah Heilshorn, Ph.D., professor of materials science and of engineering and director of the Geballe Laboratory for Advanced Materials. Lead study co-authors are former postdoctoral fellows Michael Kratochvil, Ph.D., and Sally Demirdjian, Ph.D.; and basic life research scientist Gernot Kaber, Ph.D.
A separate clinical trial led by Bollyky and recently published in the Journal of Clinical Investigation has cleared the path for further development of a drug that may be able to break the logjam.
Sputum thickness means deadly sickness
Like cystic fibrosis, severe COVID-19 is characterized by sputum—a mishmash of mucus, cellular debris, various immunologically active agents, salts and more—that’s so viscous it sticks in the lungs instead of being cleared out by the method evolution designed: coughing.
These patients are literally “drowning in their own respiratory secretions,” Bollyky said, but that accumulation is exceptionally difficult to dislodge, contributing to the infamous “dry cough” of COVID-19.
To collect sputum from severely breathing-impaired COVID-19 patients on the day they entered the intensive care unit, the Stanford researchers suctioned it out of the lungs of 17 consenting patients just after tubes were placed in their tracheas but before they were hooked up to mechanical ventilators. The patients ranged in age from 5 to 70.
“We analyzed this sputum to see what it’s made of, why it’s so difficult for the lungs to get rid of and how it affects the immune response,” Bollyky said. The investigators compared the patients’ sputum with that of 15 people whose lungs were in good health as well as with sputum from patients who had other conditions affecting the lungs, such as cystic fibrosis. In the COVID-19 patients’ sputum they found elevated amounts of three polymers, which are long sequences of small chemical units, strung together like links of a chain.
All three substances are hydroscopic—they soak up water like a sponge—and agglomerate into gelatinous tangles, impairing oxygen exchange and thickening sputum to the point at which expelling it presents what can be an insurmountable challenge.
One of the three polymeric substances the scientists showed was responsible for the pathological thickness of COVID-19 patients’ sputum was DNA, the genetic material that encodes our genes. Bollyky presumes that the high levels of free-floating DNA in COVID-19 sputum results from dead lung and immune cells’ breaking open and spilling out their contents.
A second abundant agglomeration-prone polymer in severe COVID-19 patients was mucin, a sugar-decorated protein that’s the defining substance in mucus. But mucin’s levels in severe COVID-19 patients’ sputum varied a great deal.
It was the third high-volume component of severe COVID-19 patients’ sputum—a carbohydrate (chain of sugar molecules) called hyaluronan, whose levels climbed tenfold in COVID-19 sputum compared with that of healthy controls—that raised eyebrows on the Stanford team.
“We found a ton of hyaluronan in there,” Bollyky said.
Hyaluronan (also called hyaluronic acid) is manufactured in small amounts by cells in many tissues and secreted as a structural element. Among other functions, it helps cement cells into place in intact tissues. Hyaluronan partners with collagen to form pads in our joints, like pairs of bouncy sponges that keep our bones from grinding together when we move. But it’s also produced in abundance at sites of injury and infection, drawing our immune systems’ attention and promoting inflammation.
This pro-inflammatory character becomes especially pronounced if initially lengthy sequences of hyaluronan are broken into smaller fragments in the fray. In tissues where such shorter hyaluronan shards abound—as the researchers learned they do in sputum from severe COVID-19 patients’ lungs—immune overdrive can lead to fibrosis, the formation of scar tissue. Fibrotic lungs, in turn, make for chronic shortness of breath—a symptom often reported by long COVID-19 sufferers.
Using enzymes that break down DNA and hyaluronan, Bollyky and his colleagues showed that each enzyme independently reduced the viscosity of COVID-19 patients’ sputum samples. But safety concerns preclude testing a DNA-degrading enzyme in patients. In any case, enzymes are not only expensive but finicky—they have to be handled with great care if they are to remain intact and active.
Might there be a safer small molecule that could pinch hit for the enzyme that breaks down hyaloronan, the most appealing drug target in the trio of polymeric contributors to COVID-19 lungs’ goopy gridlock? The answer may be yes.
Breaking up the logjam
A paper published May 2 in The Journal of Clinical Investigation describes a recently concluded clinical trial, led by Bollyky, of a small-molecule drug that’s been shown in lab studies to prevent the buildup of hyaluronan. This drug, 4-methylumbelliferone, or 4-MU, has never been tested for that purpose in humans.
Unavailable in the United States, 4-MU was approved in Europe half a century ago and is widely available in Asia, Africa and the Middle East—but only for treating a condition unrelated to COVID-19: It’s used to counter biliary spasm (the intense pain experienced by people with gallstones when their gallbladders, periodically contracting to squirt bile into the digestive tract, wind up squeezing the stones). 4-MU’s safety record is excellent, and it’s inexpensive because it’s off-patent. But its current formulation is not optimized to treat chronic disease. To get the U.S. Food and Drug Administration’s approval for a new therapeutic use, 4-MU must complete a full battery of clinical trials in the United States.
The phase 1 clinical trial led by Bollyky showed that not only was the existing formulation of 4-MU well tolerated at three different doses, but it also significantly lowered hyaluronan levels in the sputum of the participants, who were all healthy and started out with low circulating hyaluronan levels. The FDA has now approved further clinical tests of the drug for treating COVID-19, cystic fibrosis and other respiratory-secretion-associated disorders.
4-MU is not an antiviral. It wouldn’t compete against drugs designed to reduce viral load. But it could complement them by reducing patients’ accompanying, potentially lethal lung distress. It might also alleviate lung congestion that persists in severe COVID-19 patients after the virus has left the scene, Bollyky said. That could prevent fibrosis down the road in lungs rendered vulnerable by SARS-CoV-2 infection.
Source: Read Full Article
