Stem cell therapy restores injured tendons to full health in study on sheep

Tendon injuries in sheep that were treated with stem cell grafts achieved, in just two months, a diameter and hardness similar to the original healthy tendon, according to the results of a study released in Stem Cells Translational Medicine (SCTM). These findings suggest that the treatment, which uses autologous adipose micrografts—grafts of stem cells derived from fat taken from the recipient—presents a safe, reliable and relatively fast way to promote tendon healing.
Tendons are the fibrous tissues connecting muscle to bones. Their job is to transmit the contraction force produced by muscles to the bone they hold, thereby achieving movement. Due to overuse or age-related degeneration, tendon injuries have become a common clinical problem. Damaged tendons heal slowly, and current treatments often can’t manage the pain. They also are unable to restore the tendon’s original structure and functionality.
“Not only does the patient suffer, but the increased incidence rate and ineffective treatments of tendon and other musculoskeletal disorders have resulted in a rise of up to $874 billion from 2000 to 2015, representing an important socioeconomic burden on healthcare worldwide; hence, an effective and affordable therapeutic plan is surely imperative,” said Francesco De Francesco, M.D., a member of the Reconstructive Surgery and Hand Surgery unit at AOU “Ospedali Riuniti” (United Hospitals) of Ancona, Italy. He was co-senior author of the new study, which was a multi-institutional collaboration involving colleagues from his University as well as from the University of Camerino, University of Parma, Polytechnic University of Marche and the University of Ferrara.
In the search for new and better ways to heal injured tendons, the medical world is looking closely at regenerative therapies. In particular, autologous adipose micrografts (AAMGs) and stromal vascular fraction (SVF) are showing promise. SVF, derived from adipose tissue, contains heterogeneous cell populations such as mesenchymal progenitor/stem cells, endothelial cells, pericytes, T cells and M2 macrophages. SVF-derived mesenchymal progenitor/stem cells can be easily expanded in vitro and have the potential to create diverse lineages of cells.
In a previous study on rats, AAMGs and SVF improved tendon healing in 60 percent to 70 percent of treated animals. The purpose of this new study reported on in SCTM was to evaluate the effects of AAMG in sheep with tendinopathy, as larger animals are more comparable to humans than are rodents.
“This is also the first study on an animal model employing a mechanical fat breakdown system as an alternative to enzymatic digestion to isolate the SVF. The process consists of gently disaggregating adipose tissue using a particular micro-blade grid and a filter for cells within a sterile capsule. This device leads to the generation of a micrograft suspension that is ready for use and rich in SVF, extracellular matrix fragments and growth factors, and facilitates and enhances the regenerative potential of the isolated tissue fragments,” Dr. De Francesco explained.
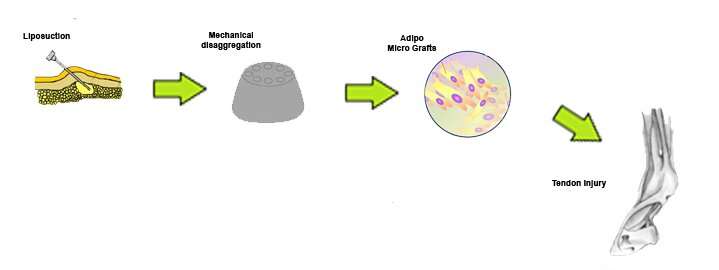
This procedure is able to maintain the microenvironment of the perivascular niche, while at the same time removing any pro-inflammatory factors. The residual SVF contain pericytes that are able to gradually convert into activated adipose stem cells.
“The resulting AAMG has a great anti-inflammatory and healing effect when applied to musculoskeletal disorders. Moreover, the harvesting procedure is easier, faster, safer, and more reliable and with less morbidity to the donor site than harvesting bone marrow or platelet-rich plasma,” he added.
The team carried out the study by inducing tendinopathy in both common calcaneal tendons (CCT) of 16 female sheep. Tendinopathy is a breakdown of collagen in a tendon, resulting in burning pain, reduced flexibility and limited range of motion.
Four animals were assigned to a non-treated group as a control. Each of the other 12 sheep had one CCT injected with AAMG, while its contralateral CCT was left untreated.
“Two-months post-inoculation, data gained from our analyses showed that in the group treated with SVF the tendon diameter and hardness were similar to that of uninjured tendons. Additionally, we observed positive effects in matrix composition in the treated tendons and in collagen deposits, as well as noted improved blood vessel formation within the lesion sites,” reported co-senior author Michele Riccio, M.D., director of the Reconstructive Surgery and Hand Surgery Unit at AOU United Hospitals of Ancona, Italy.
“Our findings suggest that the beneficial effects of tendon repair induced by SVF is attributable to the maintenance and induction of tendon fiber organization, rather than an increase in a pool of cells as part of the healing process,” he continued. “This further indicates that SVFs represent a safe, reliable and more effective treatment for tendinopathy, with a lower rate of post-intervention complications, than current therapies. We believe it strengthens the rationale for their use as a tendinopathy treatment in humans.”
Source: Read Full Article
