Study identifies chromatin alterations in the brains of patients with schizophrenia and bipolar disorder
The complete set of nucleic acid sequences composing chromosomes in humans, encoded as DNA, is known as the “human genome sequence.” Neuroscientists predict that approximately 25% of this sequence has a regulatory function, while only 1.5% encodes protein.
In recent years, many epigenetics studies have tried to identify the mechanisms through which regulatory sequences in the human genome activate or inhibit the expression of genes in response to environmental factors. This could in turn help to modify these sequences using genetic techniques, to reduce the prevalence of genetically inherited diseases or conditions.
Researchers at Icahn School of Medicine at Mount Sinai in New York have recently carried out a study specifically examining chromosomal organizations that are associated with the heritability of two major psychiatric conditions, namely schizophrenia and bipolar disorder. Their paper, published in Nature Neuroscience, unveiled a series of alterations liked to the organization of the genome in three-dimensional (3D) space in brains of patients with these two psychiatric disorders, which are known to have a similar genetic risk architecture.
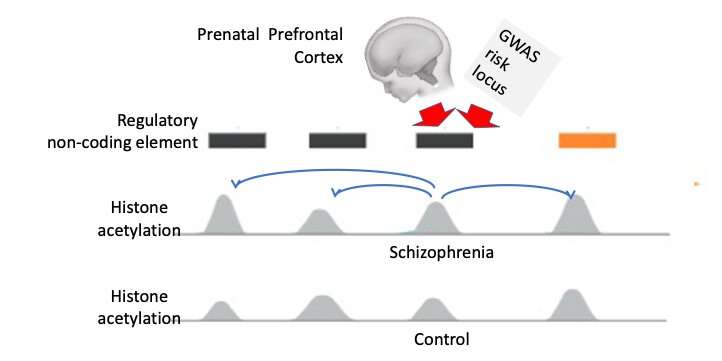
“Importantly, up to ~90% of genetic risk sequences for schizophrenia and related psychiatric illness are located in the regulatory non-coding genome,” Kiran Girdhar, one of the researchers who carried out the study, told Medical Xpress. “Many of these types of DNA sequence are ‘decorated’ with chemical signal, such as histone acetylation and some types of histone methylation, that are indicators for the DNA sequence being involved in gene expression activity.”
As part of their study, Girdhar and her colleagues Panagiotis Roussos and Schahram Akbarian sequenced 739 data libraries, each of which outlined a genome-map of histone acetylation and methylation markings that are associated with active gene expression. These libraries included the data of 388 subjects and 351 individuals either diagnosed with schizophrenia or bipolar disorder. To investigate the role of non-coding risk genetic variants in schizophrenia and bipolar disease, they profiled histone acetylation, an epigenetic alteration known to change the structure of chromatin and regulate gene expression, at a base pair resolution.
“In our study, we tried to answer three questions, which had so far been unanswered by literature our field,” Girdhar said. “Are the non-coding risk variants in schizophrenia embedded in the genome marked by our chromatin mark (histone acetylation)? Do schizophrenia and bipolar brains show a change in their histone acetylation landscape and if so, are these changes linked to the genetic risk architectures of these psychiatric conditions? And finally, how are acetylated chromatin domains organized in 3D space inside the nucleus of brain cells?”
Using the large human genome dataset they compiled, Girdhar and her colleagues were able to map thousands of cis-regulatory domains (i.e., genome sequence elements with a transcriptional regulatory function), revealing a fine-grained chromosomal organization. Their analyses unveiled a series of chromatic-related alterations in the 3D genome organization of both patients diagnosed with schizophrenia and bipolar disorder.
“By leveraging our large-scale histone dataset, we estimated inter-individual correlation to identify physically interacting chromosomal loci in the 3D genome, each locus marked by acetyl-histone,” Girdhar said. “Clusters of ‘active’ non-coding parts of the linear genome marked by histone acetylation represent building blocks of 3D structure of the genome.”
Using these “building blocks,” the researchers showed that many chromatin domains with disordered acetylation in the brains of patients diagnosed with schizophrenia or bipolar disorder were commonly located deep inside the nerve cell nucleus in ‘active’ compartments. These were compartments with high levels of gene expression.
“These types of clusters of acetylated chromatin domains were hyperacetylated in our schizophrenia and bipolar brains in comparison to controls,” Girdhar said. “We speculate that these parts of the epigenome could be the ‘seed’ points that ultimately spread to a wider part of the genome inside the neuronal nucleus.”
If the researchers’ predictions are correct, early genetic interventions on these sections of the epigenome could correct the “faulty” chromatin acetylation that they identified before the disorders becomes more severe or chronic. In the future, their study could thus potentially pave the way towards more effective early interventions for schizophrenia and bipolar disorders.
“The dysregulated clusters in SCZ brains were enriched in glutamatergic neurons whereas hypo-acetylated clusters were enriched in gabaergic neurons in SCZ brains,” Girdhar said. “Interestingly, we observed a subset of hyper-acetylated clusters in active compartments in neurons, were linked to neurodevelopment and had significant enrichment for SCZ common genetic variants in SCZ brains.”
Future studies could investigate the subset of hyper-acetylated clusters that Girdhar and his colleagues observed in active neuronal compartments further. This could shed light on the specific stages of neurodevelopment that might be disrupted in the brain of patients diagnosed with schizophrenia and bipolar disorder.
Source: Read Full Article
