Study looks at Achilles heel of insulin pump technology
Since the insulin pump started widespread use in the early 1980s, it’s become the option of choice for type 1 diabetes patients to manage their glucose levels in a way that doesn’t require testing their blood sugar and injecting insulin multiple times daily.
But now, a first-of-its kind study is looking at the issue of patients “running out of real estate” due to pump sites becoming fibrotic, irritated and less effective at delivering insulin. The UW Medicine-led study was published July 14 in the journal Diabetes Care.
“No one had done a human study on what happened to the skin under these sites until now,” said Dr. Irl Hirsch, professor of medicine, Division of Metabolism, Endocrinology, and Nutrition at the University of Washington School of Medicine. He is also the diabetes treatment and teaching chair in the Department of Medicine.
Hirsch estimated that well over 70% of the patients with type 1 diabetes seen at the UW Medicine Diabetes Institute are on insulin pump therapy. The advances in insulin pump therapy have freed up patients from the daily routines of injections and, when connected to continuous glucose monitors, can give them precise dosing based on their blood glucose levels. However, there is an Achilles’ heel of the therapy that has not been addressed, said Hirsch.
“It really doesn’t matter how good the technology is,” he said. “We still don’t understand what is happening with the infusion sites, much less fix it.”
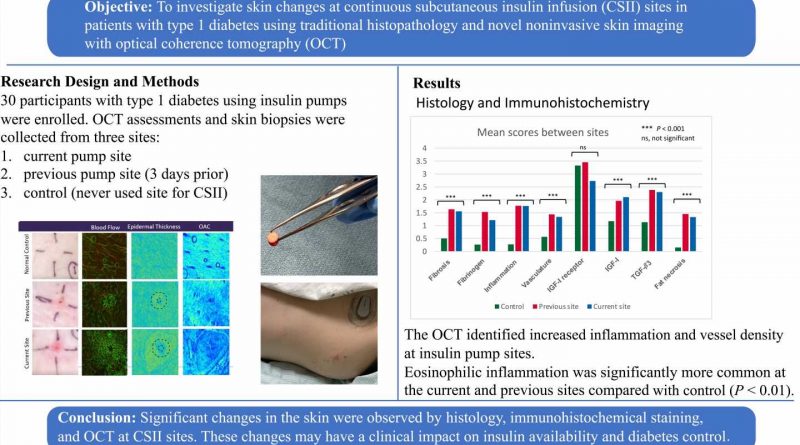
This study, performed between 2020 and 2022 (paused due to the pandemic) enrolled 30 participants from the UW Medicine Diabetes Institute. It was the first such step to answer both these questions.
The study participants were divided into two groups: those patients using insulin pumps for 10 years or less, and those using pumps for over 20 years. Researchers expected to see more pathology—thickening of skin, damage to the subdermal layer, inflammation—in the group using the pumps for 20 years or more. That’s not what happened.
“We found that the pathology, to our surprise, was no different when short-term user results were compared with long-term users,” he said.
Both groups had high levels of eosinophils, disease-fighting white blood cells that usually appears in the blood to fight allergies. Generally, they assist in healing the skin and creating fibrosis.
“This is the last thing you want at an infusing site,” Hirsch said.
Using a non-invasive technique, called optical coherence tomography, or OCT, researchers were able to monitor blood flow and inflammation around the sites. Greater blood flow would result in quicker insuln absorption. Skin biopsies were taken at the pump infusion sites.
“From a bigger point of view of fibrosis, inflammation and eosinophils, we saw all this in both groups, but we don’t understand yet why it’s happening,” he said. “How much was the catheter or the insulin causing the irritation around the sites? How much was it from the preservatives or is this because of the insulin pump itself?”
In addition, some patients move the injection site from place to place because of irritation, and other patients have no irritation at all. Yet, researchers don’t know why.
All these questions need to be answered in future studies, he said.
“Ninety-three percent of those in the study complained of itching, which points to eosinophils being present, but we are also going to look at metabolomics,” he said. “The real goal of all of this is to minimize skin damage and improve the experience for our patients.”
More information:
Andrea Kalus et al, Evaluation of Insulin Pump Infusion Sites in Type 1 Diabetes: The DERMIS Study, Diabetes Care (2023). DOI: 10.2337/dc23-0426
Journal information:
Diabetes Care
Source: Read Full Article