Synthetic molecule proves able to mitigate heart failure in translational study
Researchers at the University of São Paulo (USP) in Brazil, partnering with Foresee Pharmaceuticals, a Taiwan and US-based biopharmaceutical company, have tested a synthetic molecule for the treatment of heart failure. The study has been published in the European Heart Journal. The theme was also highlighted in the magazine’s editorial.
Heart failure is a condition in which the heart muscle cannot pump enough blood to meet the body’s needs for blood and oxygen. It causes more deaths worldwide than any other disease, in the sense that other cardiovascular disorders tend to lead to heart failure, which affects over 2 million people in Brazil. A number of drugs can slow its progression, but currently no treatment exists that can reverse it even partially. A heart transplant is considered if the condition becomes severe.
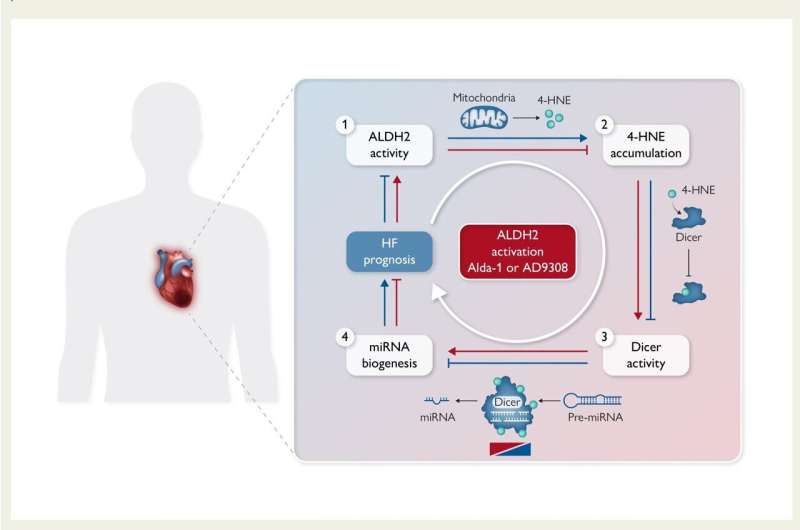
In the translational study conducted at USP, several experiments were conducted to demonstrate the effect of the molecule, named AD-9308, to restore the activity of the enzyme aldehyde dehydrogenase 2 (ALDH2), which is present in mitochondria (the organelles that generate energy to power cells’ biochemical reactions) and plays a key role in heart failure.
“The study lasted more than ten years and included both laboratory experiments and samples from heart failure patients with the aim of understanding a novel mechanism involved in heart failure progression. In parallel with our experiments, the biopharmaceutical company worked on improving the efficacy of a molecule described back in 2014, which has potential to treat cardiac diseases,” said Julio Cesar Batista Ferreira, corresponding author of the article and a professor at the Institute of Biomedical Sciences (ICB-USP).
The molecule prototype compound first described was named Alda-1. At the time, the researchers found that the drug compound increased heart function by 40% in rats with heart failure and that this effect was due to activation of ALDH2 in cardiac cells.
Structural modifications were made to the original molecule to boost its pharmacological properties and qualify it as a potential development compound. After many versions iterations and tests, the scientists at Foresee Pharmaceuticals developed the AD-9308. “This new version activates the enzyme ALDH2 three times more than the original molecule,” Ferreira said.
The biopharmaceutical firm has completed clinical trials of another molecule similar to AD-9308, assuring its safety.
“The results show that the synthetic molecule is well tolerated by healthy subjects. These are the required steps to apply to the FDA for permission to test the drug in heart failure patients. However, it requires more volunteers and more time, but it’s the only way to find out which kind of heart failure it could treat and at what stage of the disease,” Ferreira explained. The FDA is the United States Food and Drug Administration, responsible for protecting public health by assuring the safety and efficacy of human and veterinary drugs, among other duties.
Mitochondrial malfunction
Several studies conducted at ICB-USP in the past ten years have shown that heart failure is associated with mitochondrial malfunction. Like a car engine, mitochondria convert chemical energy into mechanical energy, which the heart needs to pump blood. “When a car engine isn’t running properly, energy conversion is impaired, efficiency drops, and pollution increases,” Ferreira said.
The “pollutant” produced by mitochondria in people with heart failure is 4-hydroxynonenal, a compound belonging to the class of aldehydes.
“Every cell has hundreds or sometimes thousands of mitochondria, which produce enough aldehyde to poison the entire cell when they aren’t running properly. We discovered in this latest study that too much of 4-hydroxynonenal switches off a vital event for the cell: processing of microRNAs [small non-coding RNAs that regulate the activity of other genes],” Ferreira said.
Using mass spectrometry, the researchers observed that 4-hydroxynonenal [the aldehyde produced by mitochondria] binds irreversibly to Dicer [a protein essential to microRNA formation] and inactivates it. “In addition, we showed that AD-9308 improves mitochondrial filtration enough to eliminate this cellular pollutant,” Ferreira said.
Animals genetically engineered to lack Dicer are known to develop heart failure.
“In this study, we identified the chemical alterations that inactivate Dicer in rodents and humans owing to the accumulation of aldehyde caused by heart failure. This was a hitherto unknown mechanism. The point is that Dicer is a limiting enzyme for formation and maturation of the microRNAs responsible for overall control of cellular biology,” Ferreira said. Interruption of microRNA formation and maturation is associated with several health problems, including cancer, metabolic syndrome, neurodegenerative disorders, and cardiovascular disease.
In experiments involving animals, cell cultures and heart tissue samples from the Heart Institute (Incor) at Hospital das Clínicas, the hospital complex run by the university’s Medical School (FM-USP), the researchers found that aldehyde binding makes Dicer stop working and reduces the amount of microRNAs available to the heart.
In addition to their discovery of this novel mechanism associated with heart failure, the researchers showed in samples of human heart tissue that the disorder can be reversed, and Dicer activity restored using the drug AD-9308.
“In summary, AD-9308 stimulates the removal of aldehyde from sick cells, reducing the likelihood that it will ‘switch off’ Dicer and hence protecting the heart cells. This tends to keep the microRNA profile closer to that of a healthy heart. I consider our partnership with Foresee Pharmaceuticals a success. It’s been essential to our multidisciplinary multicenter research and development, producing highly promising results that can now be trialed in patients,” Ferreira concluded.
More information:
Ligia A Kiyuna et al, 4-Hydroxynonenal impairs miRNA maturation in heart failure via Dicer post-translational modification, European Heart Journal (2023). DOI: 10.1093/eurheartj/ehad662. academic.oup.com/eurheartj/adv … artj/ehad662/7343271
Joost PG Sluijter, Post-translational modifications upon mitochondrial dysfunction in heart failure, (2023). DOI: 10.1093/eurheartj/ehad710
Journal information:
European Heart Journal
Source: Read Full Article