Women who are pregnant or nursing should receive a COVID booster vaccination
In a recent study posted to the medRxiv* preprint server, researchers evaluated the impact of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) booster vaccination in nursing and pregnant women.
 Study: COVID-19 booster dose antibody response in pregnant, lactating, and nonpregnant women. Image Credit: HTeam
Study: COVID-19 booster dose antibody response in pregnant, lactating, and nonpregnant women. Image Credit: HTeam
Background
Pregnant women are notably sensitive to coronavirus disease 2019 (COVID-19) since they have a higher chance of severe illness and undesirable pregnancy outcomes, such as stillbirth. Despite the American College of Obstetricians and Gynecologists (ACOG) and the Centers for Disease Control and Prevention (CDC) recommendations for vaccinating pregnant women, their SARS-CoV-2 vaccination coverage is lesser than the general adult population.
Prior reports showed that the SARS-CoV-2 messenger ribonucleic acid (mRNA) vaccines induced robust immunogenicity in nursing and pregnant women. Nevertheless, the thorough characterization of the immune responses to the primary COVID-19 vaccination in lactating and pregnant women revealed diminished fragment crystallizable (Fc) receptor binding and variations in subtype preference. These data suggest the delayed advancement of a completely mature immune response against SARS-CoV-2 in the nursing and pregnant cohorts.
The introduction of booster doses of the COVID-19 mRNA vaccines was inspired by the quick waning of vaccination-induced immunity and the emergence of SARS-CoV-2 variants of concern (VOCs), like Omicron. However, it is unclear if all groups, particularly nursing and pregnant women, will respond to a booster shot in the same way.
About the study
In the current work, the researchers examined humoral immunity elicited by the booster dose of SARS-CoV-2 mRNA vaccines in 63 subjects comprising breastfeeding, pregnant and age-matched nonpregnant females. They studied the antibody response to the spike (S) proteins of the SARS-CoV-2 Omicron and ancestral strains in a group of 12 nursing, 31 pregnant, and 20 nonpregnant age-matched volunteers who received an mRNA-1273 or BNT162b2 booster dose after completing the primary COVID-19 vaccination. Further, the researchers analyzed the transmission of vaccine-triggered antibodies in 15 maternal-cord pairs at delivery.
The eligible subjects were lactating, pregnant, and nonpregnant women aged 18 to 45 years. Moreover, all included participants were vaccinated with SARS-CoV-2 mRNA booster dose between August and December 2021. The participants were selected from two tertiary care hospitals by practitioners or were self-referred. Blood samples were taken from all volunteers four weeks following the booster dose vaccination and at delivery in pregnant women. Furthermore, umbilical cord and maternal blood were obtained during delivery for 15 women who gave birth during the research period.
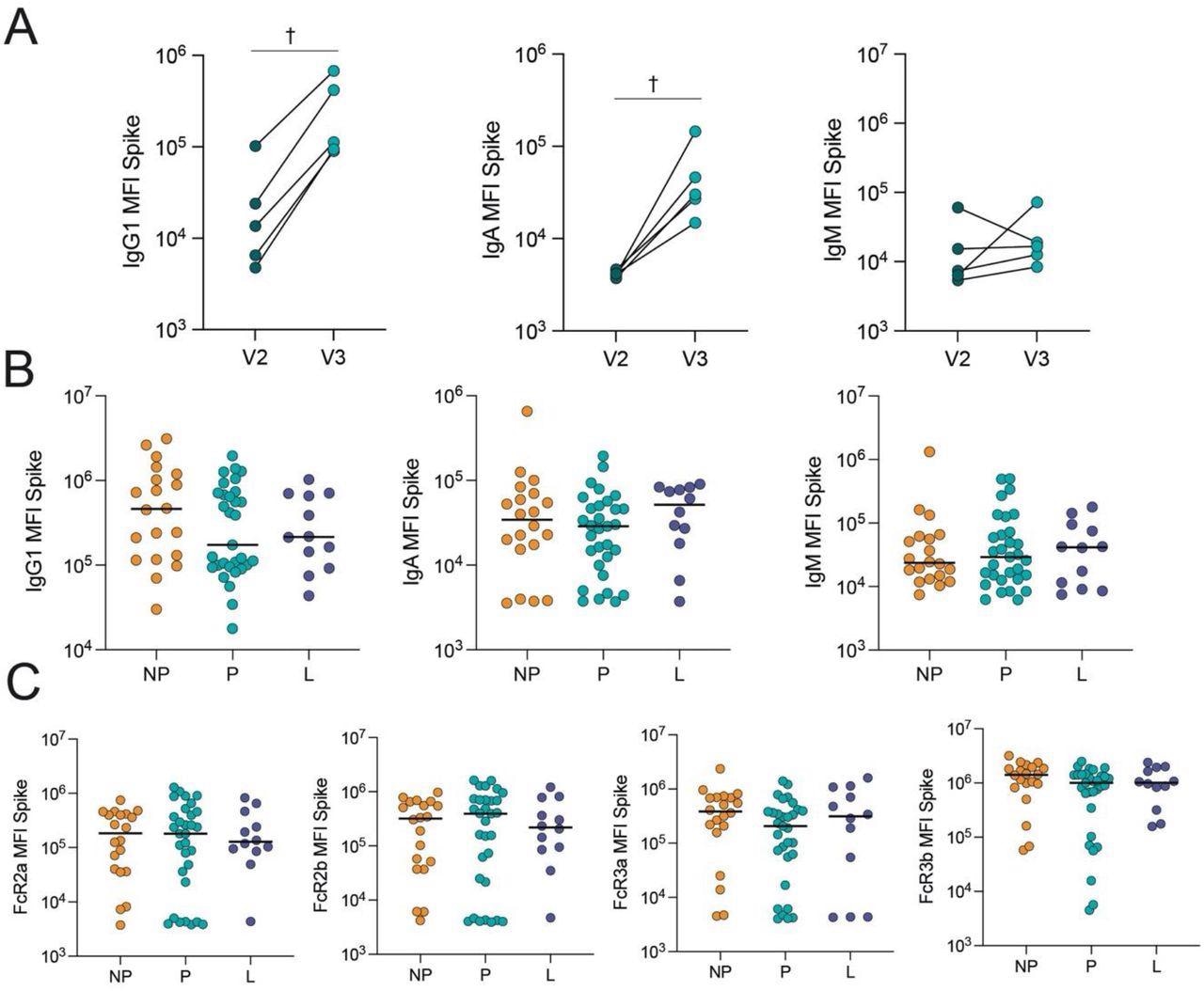
COVID-19 booster vaccine induces a similar Spike-specific antibody response in pregnant, lactating and nonpregnant individuals. A. The dot plots show the peak IgG1, IgA and IgM response against Spike in 5 pregnant individuals after receiving the second dose of a primary mRNA vaccine series (V2) and after the booster dose (V3). Lines connect samples from the same individual. Significance was determined by a Wilcoxon signed-rank test. Differences did not reach statistical significance. † p = 0.06. B. The dot plots show IgG1, IgA and IgM levels against Spike in nonpregnant (NP), pregnant (P) and lactating (L) individuals. Horizontal line represents the median for each group. Significance was determined by a Kruskal-Wallis test. No comparisons were significant. C. The dot plots show the FcR-binding of antibodies against Spike in nonpregnant (NP), pregnant (P) and lactating (L) individuals. Horizontal line represents the median for each group. Significance was determined by a Kruskal-Wallis test. No comparisons were significant.
Results
The study results showed that COVID-19 booster vaccination in pregnancy elevated Omicron S-specific immunoglobulin G1 (IgG1) levels. Lactating and pregnant women had SARS-CoV-2 Omicron and ancestral S-specific total IgM, IgA, and IgG1 levels and neutralizing concentrations similar to nonpregnant women after boosting.
Multivariate analysis indicated pregnancy-specific changes in antibody class switching and SARS-CoV-2 S-specific epitope coverage. The immune response to a booster shot in pregnant relative to nonpregnant women showed subtle variations in Fc region-receptor binding and antibody subclass patterns. In particular, functional antibodies in nonpregnant women had a greater specificity for the SARS-CoV-2 S2 domains and N-terminal domain (NTD), which might be important in the overall immune reaction to the S antigen. Surprisingly, the current findings revealed that breastfeeding women had a profile in-between nonpregnant and pregnant women.
Assessment of cord and maternal antibody patterns at delivery following third-trimester booster vaccination depicted similar total SARS-CoV-2 S-specific IgG1 in the umbilical cord and maternal blood. Further, S-specific Fc-γ-resistance gene (R3a)-binding antibodies were higher in the cord blood than in maternal blood, indicating that highly efficient IgG was transferred preferentially. The authors found that the time following receiving the booster shot was positively linked with the SARS-CoV-2 S-specific IgG1 concentrations in the cord.
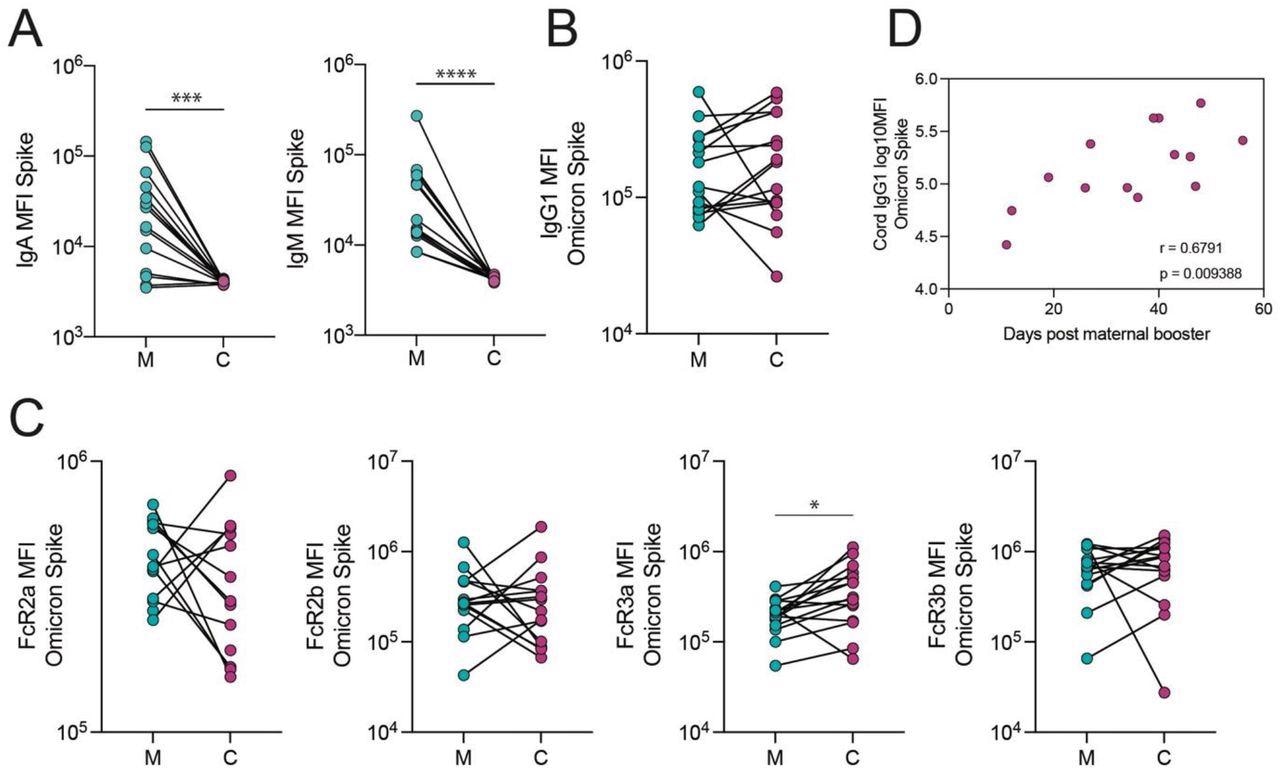
The transfer of Omicron Spike-specific antibodies to the cord after vaccination in the third trimester. A. The dot plots show IgA and IgM against Spike in maternal (M) and cord blood (C). Lines connect matched maternal:cord dyads (n=15). ***p<0.001, ****p<0.0001. B-C. The dot plots show the IgG1 (B) and FcR-binding (C) titer against Omicron Spike in maternal (M) and cord blood (C). Lines connect matched maternal:cord dyads (n=15). Significance was determined by a Wilcoxon signed-rank test followed by a Benjamini-Hochberg correction for multiple testing, * p<0.05. D. The scatter plot shows the correlation of log-transformed cord IgG1 levels against Omicron Spike versus days from maternal booster to delivery. R value reflects a Spearman correlation.
Conclusions
The study findings implied that receiving a COVID-19 mRNA vaccination booster dose during pregnancy results in a robust SARS-CoV-2 S-specific humoral immunity, including protection against the Omicron VOC, in neonates and mothers equivalent to nonpregnant females. Furthermore, the scientists stated that if SARS-CoV-2 vaccine boosting happens in the final trimester, S-specific umbilical cord IgG1 titers will be higher as antibody transferring occurs in a time-reliant manner.
Overall, the present work indicated that a COVID-19 vaccine booster shot in pregnant ladies might help to enhance the immunity of newborns and mothers against SARS-CoV-2 infection. In addition, the authors mentioned that it is essential to assess whether more booster doses or different vaccination platforms could increase epitope coverage in pregnancy and break the SARS-CoV-2 receptor-binding domain (RBD) immunodominance.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- COVID-19 booster dose antibody response in pregnant, lactating, and nonpregnant women; Caroline Atyeo PhD, Lydia L Shook MD, Nadege Nziza PhD, Elizabeth A DeRiso PhD, Cordelia Muir, Arantxa Medina Baez, Rosiane S Lima, Stepan Demidkin, Sara Brigida, Rose M De Guzman PhD, Madeleine D Burns, Alejandro B Balazs PhD, Alessio Fasano MD, Lael M Yonker MD, Kathryn J Gray MD PhD, Galit Alter PhD, Andrea G Edlow MD MSc. medRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.05.17.22275154, https://www.medrxiv.org/content/10.1101/2022.05.17.22275154v1
Posted in: Child Health News | Medical Research News | Women's Health News | Disease/Infection News
Tags: Antibodies, Antibody, Antigen, Blood, Breastfeeding, Coronavirus, Coronavirus Disease COVID-19, covid-19, Gene, Immune Response, immunity, Immunoglobulin, Nursing, Omicron, Pregnancy, Receptor, Research, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Stillbirth, Syndrome, Umbilical Cord, Vaccine

Written by
Shanet Susan Alex
Shanet Susan Alex, a medical writer, based in Kerala, India, is a Doctor of Pharmacy graduate from Kerala University of Health Sciences. Her academic background is in clinical pharmacy and research, and she is passionate about medical writing. Shanet has published papers in the International Journal of Medical Science and Current Research (IJMSCR), the International Journal of Pharmacy (IJP), and the International Journal of Medical Science and Applied Research (IJMSAR). Apart from work, she enjoys listening to music and watching movies.
Source: Read Full Article
