cheapest brand cialis de next day
<img class="aligncenter" src="https://scx1.b-cdn.net/csz/news/800a/2023/skin-cancer-rewires-it.jpg"
alt="Skin cancer rewires its energy systems to spread more efficiently"
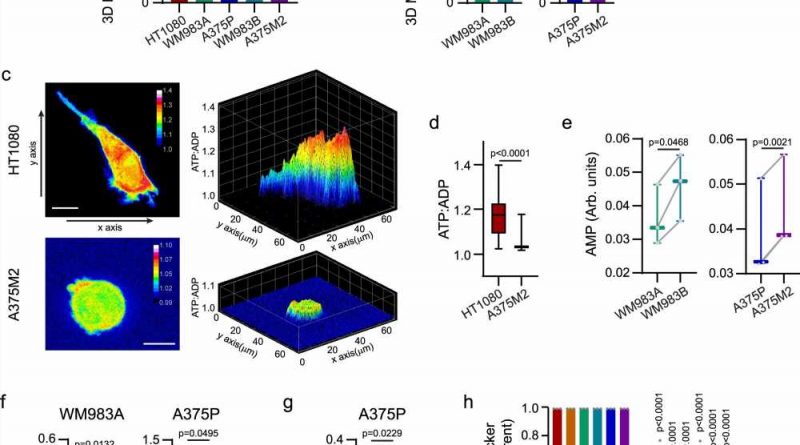
title="Different modes of migration and mitochondrial metabolism. a, b Oxygen consumption rate (OCR) from cells embedded in a 3D collagen I matrix (n = 3). Comparison between elongated-mesenchymal and rounded-amoeboid cells in a panel of cell lines and between the melanoma pairs. c Maximal intensity projection (left) and 3D representation (right) of ATP:ADP ratio in HT1080 and A375M2 cells expressing Perceval HR biosensor, embedded in a 3D collagen I matrix. Scale bar = 10 μm. d Quantification of ATP:ADP ratio from (c) (18 cells/condition pooled from n = 3). e AMP levels quantified by 1H-NMR for the indicated cell lines (n = 3). f Intracellular ATP levels in WM983A and A375P cells grown under adherent or floating conditions for 24 h (WM983A, n = 4; A375P, n = 5). g XF ATP rate index indicative of the ratio of mitochondrial and glycolytic ATP Production Rate in A375P under adherent or floating conditions for 24 h (n = 4). h Quantification of Tetramethylrhodamine Ethyl Ester Perchlorate (TMRE) fluorescence intensity normalized by MitoTracker Deep Red signal by FACS in cells adhered on plastic or floating cells for 24 h. Data shown as fold versus adherent cells (n = 3). Graphs (a, b, toprol pain medication f, g, h) show mean ± SEM. Box plots (d, e) show median (center line), interquartile range (box) and min-max values (whiskers). p values were calculated using two-tailed tests (a, b, d–g). p value by unpaired t-test comparing elongated-mesenchymal versus rounded-amoeboid cells (a), unpaired t-test (b), paired t-test (e–g), Mann-Whitney test (d) and two-way ANOVA with Sidak’s correction comparing floating vs adherent cells (h). All n are indicative of independent experiments unless otherwise stated. Credit: Nature Communications (2023). DOI: 10.1038/s41467-023-38292-0″ width=”800″ height=”530″>
Melanoma skin cancer cells radically rewire their internal power systems to drive their spread to other parts of the body, a new study shows.
The research, led by investigators at Barts Cancer Institute, Queen Mary University of London, suggests that reversing this change can make tumor cells less invasive. The team also identified a key molecule that orchestrates this process—knowledge that could lay the foundations for new therapeutic strategies to halt the spread of cancer.
Cancer cells’ ability to break away from the original tumor and spread to other parts of the body presents one of the greatest challenges to treating the disease. This process, called metastasis, seeds secondary tumors that grow in other organs, ultimately causing most cancer deaths.
“We’re still not targeting the secondary disease enough in the clinic, and I think we need to change this,” comments Professor Victoria Sanz-Moreno, lead author of the new study. “In our lab, we want to understand: what are the characteristics of cells that are able to metastasize? What are their weaknesses? And how do we target them?”
Melanoma skin cancer is among the quickest-spreading cancer types and is a key focus of Professor Sanz-Moreno and her laboratory’s research. If melanoma is diagnosed at an early stage before it spreads, almost all patients in the UK survive their disease for a year or more. But this survival drops to just over half once the disease has spread. The team’s work aims not only to equip us with the knowledge to better treat melanoma but also to unlock an improved understanding of how all cancers spread.
In the new study, published in Nature Communications, the team investigated how metastasizing cells rewire their energy systems to move quickly and efficiently on their journey to other parts of the body.
The researchers examined migrating tumor cells in a special model system allowing movement in three dimensions—a departure from conventional systems that place cells on a flat surface that doesn’t accurately replicate how cells move through living tissue. They found that metastasizing tumor cells adopt a style of movement known as rounded-amoeboid migration, where cells maintain a loose connection to their surroundings, enabling them to slither through the tissue.
This requires far less energy than a common style of cell movement known as mesenchymal migration, where cells grip tightly onto their surroundings and drag themselves through their environment.
They observed that the invasive tumor cells reshape their mitochondria to suit this efficient style of movement, opting to have many, small, fragmented mitochondria that operate in a low-power mode. This is in contrast to less-invasive cells, which have large, branching networks of mitochondria that operate in a high-power mode.
“These metastatic cells are rewiring themselves to be very efficient,” explains Dr. Eva Crosas-Molist, first author on the new paper. “They only need low levels of energy to move, which helps them to survive in the potentially stressful environments they are migrating to, where there may be a lack of nutrients or oxygen.”
Intriguingly, the team found that if they manipulate the shape of the mitochondria in their metastasizing tumor cells and force them to become more joined up, the cells lose their invasive behavior. Likewise, if they make mitochondria more disconnected in non-invasive cells, the cells start to behave like metastasizing tumor cells. The researchers discovered that a molecule called AMPK sits at the center of these processes. It senses the energy requirements of the cell and also controls the cytoskeleton, which determines how the cell moves and behaves.
“That was a really surprising thing for us—we wouldn’t have imagined that changing the mitochondria could affect the cytoskeleton and vice versa.” Professor Sanz-Moreno explains. “By modifying these little mitochondria you create a global change, altering what the cell looks like and its whole behavior.”
Professor Ketan Patel, Chief Scientist at Cancer Research UK, said, “Patients whose cancer has spread often face tougher treatments and lower chances of survival. These insights about how cancer cells travel around the body could be incredibly valuable for designing interventions to prevent this in the future. The more we know about what’s happening in the bodies of people with cancer, the greater our ability to tackle it will be.”
More information:
Eva Crosas-Molist et al, AMPK is a mechano-metabolic sensor linking cell adhesion and mitochondrial dynamics to Myosin-dependent cell migration, Nature Communications (2023). DOI: 10.1038/s41467-023-38292-0
Journal information:
Nature Communications
Source: Read Full Article