flagyl ohio
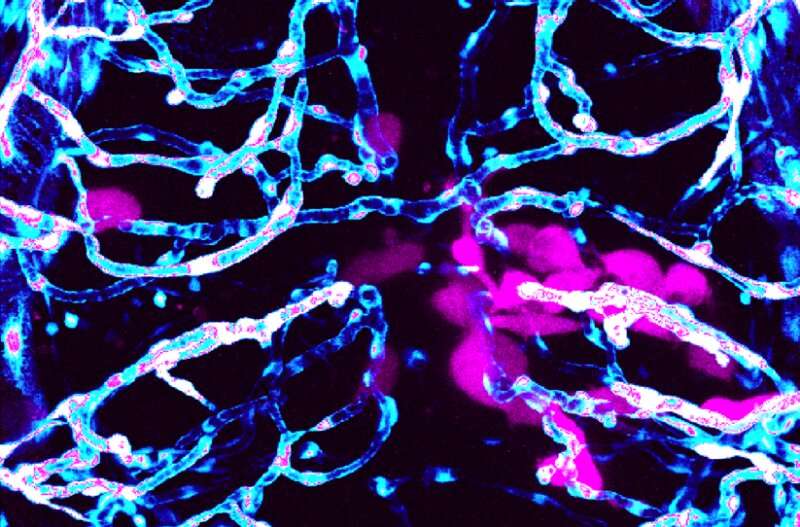 buy vytorin coupons no prescription and blood vessels, stained in blue and white, just two days after the human tumor cells were implanted in the fish. Credit: Robyn Umans, Sontheimer Lab” width=”800″ height=”527″>
buy vytorin coupons no prescription and blood vessels, stained in blue and white, just two days after the human tumor cells were implanted in the fish. Credit: Robyn Umans, Sontheimer Lab” width=”800″ height=”527″>
Scientists at the Fralin Biomedical Research Institute at VTC have identified a new zebrafish model that could help advance glioblastoma multiforme research. Glioblastoma is an aggressive form of primary brain tumor—fewer than one in 20 patients survive five years after diagnosis.
The research team previously discovered that human-derived brain cancer cells in mice use the brain’s blood vessels like highways to spread away from the original mass. In the new study, published in ACS Pharmacology and Translational Science, they show clear cross-over between mammals and fish and describe similar observations in zebrafish.
“Our hope is that this new work in zebrafish will help researchers efficiently evaluate new, much-needed therapeutics in a pre-clinical animal model to target these aggressive and devastating tumors,” said Harald Sontheimer, formerly a professor at the Fralin Biomedical Research Institute at the time of the study.
When brain cancer cells disperse, they become harder to locate and delete. Even when the original tumor is surgically removed, migratory glioblastoma cells can linger—undetected by diagnostic imaging—eventually forming new satellite tumors if they can endure chemotherapy or radiation therapies.
As the cancer cells migrate, they also leave a destructive wake.
“Our previous research showed that glioma cells exploit the brain’s blood vessels. Tumor cells use blood vessels as a pathway to invade within the brain and consequently break down the blood-brain barrier,” said Robyn Umans, postdoctoral associate in Sontheimer’s laboratory at the Fralin Biomedical Research Institute and the study’s lead author.
But what if there were a way to block glioma cells from leveraging blood vessels to control cancer cell migration?
Rodent studies led by other researchers revealed that gliomas need a key signaling pathway—Wnt, named for the wingless flies it was first observed in—in order to exploit blood vessels. When Umans added a Wnt inhibitor molecule to the zebrafish’s water, they noticed a change in glioma cell behavior—the cancer cells had reduced attachments to the brain’s vasculature.
Zebrafish skin and scales are crystal clear. Scientists can put the fish under a microscope and, with fluorescent markers, watch cancer cells grow, migrate, and interact with other cells—all in real-time.
Fast-growing zebrafish are also efficient, less expensive to maintain than other preclinical models, and they share 70 percent of the same DNA as humans.
“For me, seeing is believing. With zebrafish models I can see biological mechanisms underlying aggressive cancers come to life in real-time, gaining this unprecedented visibility into the tumor microenvironment,” Umans said. “Our hope is that these proof-of-principal experiments validate the zebrafish model as an additional platform for cancer drug discovery.”
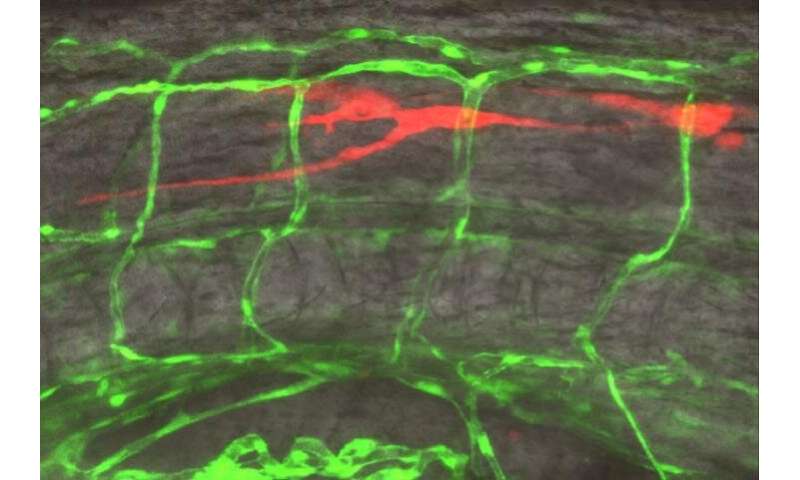
The researchers also wanted to see if the glioma cells also metastasized using blood vessels outside of the brain, so they injected a handful of cells into the fish’s peripheral tissue. This group of cancer cells attempted to travel to the brain, but they didn’t latch onto any pre-existing blood vessels. Instead, nearby vessels appeared to stem offshoots that grew toward the cancer cells like a magnet, suggesting angiogenesis, the growth of new blood vessels—a hallmark of many cancers that helps assure steady nutrient supply.
Source: Read Full Article
