functional medicine raleigh durham
Scientists at Scripps Research, with collaborators in Japan, have discovered how a “poisoned” form of a protein could set off a cascade of events that encourage the growth of some cancers. The research, published in Nature Communications on February 4, also triggered development of a drug candidate that can revert the protein to its normal form. In mice with colon cancer, diazepam codeine effects the drug prevented or dramatically slowed formation of tumors.
“This is a potentially very important and druggable link between the environment, genes and cancer,” says senior author Stuart Lipton, MD, Ph.D., professor and Step Family Foundation Endowed Chair in the Department of Molecular Medicine at Scripps Research and a clinical neurologist in La Jolla, Calif.
The study was a collaboration with a team led by Takashi Uehara at Okayama University in Japan.
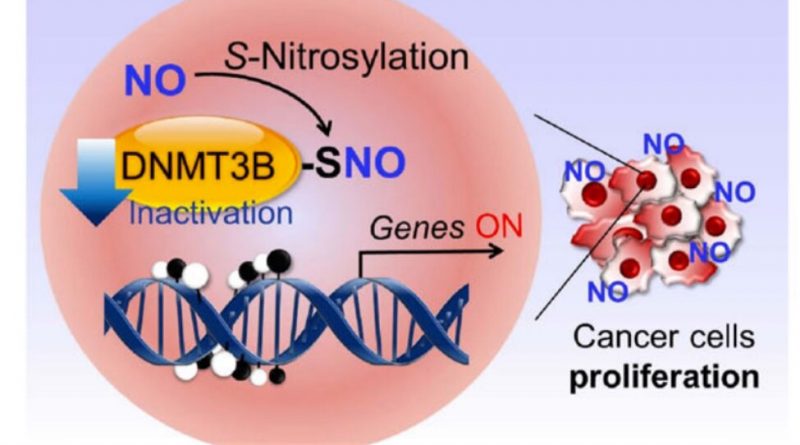
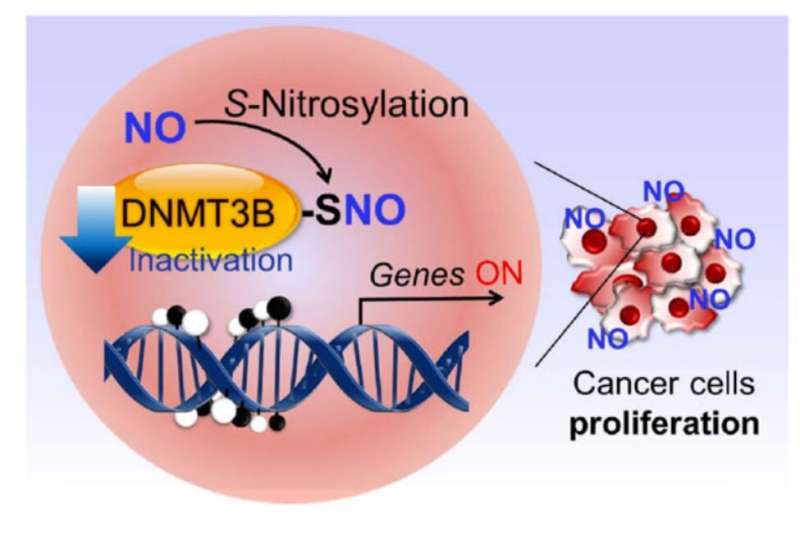
Lipton’s research group previously discovered a process called protein S-nitrosylation, in which a molecule related to nitric oxide (NO) binds to sulfur atoms within proteins to change those proteins’ functions. NO is found naturally within the body and produced in response to inflammation. But it also can form from nitrates and nitrites that are eaten (in the form of processed meats) or breathed in (through cigarette smoke or air pollution). Recently, the team showed how S-nitrosylation might contribute to Alzheimer’s disease, as well as Parkinson’s disease, Lewy body dementia, Lou Gehrig’s disease (ALS) and some forms of autism.
Separately, scientists know that many genes can be turned on or off by proteins called DNA methyltransferases (a process known as epigenetic control of gene expression). When these proteins add a methyl group—a kind of chemical marker—to a strand of DNA, they keep nearby genes from being activated. In some cancers, those methyl “silencers” are removed, and genes involved in tumor growth and spread get abnormally turned on.
“If you block methylation, genes get turned on when they shouldn’t be, and that’s been known to be an important driver of some cancers,” says Lipton. “But no one knew the prime trigger for this process.”
In the work, Lipton, with Scripps Research investigator Tomohiro Nakamura and their colleagues in Japan, showed that when DNA methyltrasferase 3B (DNMT3B) is S-nitrosylated—which can happen in the presence of high levels of NO—it no longer adds methyl groups to DNA. This then enables certain those cancer-causing genes to turn on. The findings suggest one way that processed meats, air pollution, cigarette smoke and inflammation—all linked to some forms of cancer—could flip DNMT3B to its cancer-promoting form.
“It’s like a poisoned form of DNMT3B,” says Lipton.
The group went on to show that when DNMT3B is “poisoned” in this way, expression levels of 173 different genes in human cells changed. Among these genes is Ccnd2, which was already known to be involved in the formation of gastric and colon cancers in humans.
The research group in Japan then designed a drug that would prevent DNMT3B from being S-nitrosylated, but not block its normal function or affect the S-nitrosylation of any other proteins. This prevented NO, even when present at high levels, from converting DNMT3B into the “poisoned” form.
Lipton and Uehara’s teams found that the drug, known as DBIC, prevented isolated precancerous colon cells from turning into full-blown colon cancer in the lab. Moreover, when they gave DBIC to mice prone to colon cancer, the drug virtually prevented tumors from forming, even when inflammation produced high levels of NO.
The researchers think that the S-nitrosylation of DNMT3B is likely associated with other cancers, including brain and breast cancer. They’re planning more research on the full list of genes that are impacted by S-nitrosylated DNMT3B.
“We still don’t know the full panoply of tumor types that this molecular switch might be associated with,” says Lipton. “We’ll be pursuing that in the future, as well as trying to move DBIC toward human clinical trials.”
More information:
Kosaku Okuda et al, Pivotal role for S-nitrosylation of DNA methyltransferase 3B in epigenetic regulation of tumorigenesis, Nature Communications (2023). DOI: 10.1038/s41467-023-36232-6
Journal information:
Nature Communications
Source: Read Full Article