generico claritin d xarope
<img class="aligncenter" src="https://scx1.b-cdn.net/csz/news/800a/2023/researchers-uncover-ne-9.jpg"
alt="Researchers uncover new targets for breast cancers resistant to standard therapies"
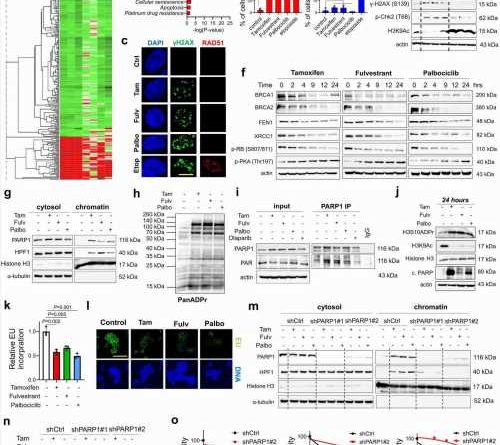
title="SOC therapy commonly induces DNA damage, BRCAness and toxic PARP1 trapping, leading to transcriptional blockage and growth inhibition in ER+ breast cancer. a Heatmap of commonly differentially expressed genes in MCF-7 cells treated with SOC (tamoxifen or fulvestrant or palbociclib) for 24 hours from the Connectivity Map database. Green: downregulated genes; red: upregulated genes. b The pathway enrichment analysis of the SOC sensitivity signature. c IF staining of γ-H2AX (S139) (green) and RAD51 foci (red) in T47D cells upon treatment with SOC for 4 hours. DAPI (blue) was used to stain the nucleus, here and in all relevant figures. Etoposide was used as a positive control. Scale bar = 100 µm. d The quantification of γ-H2AX positive cells (left) and those that are also RAD51 foci positive (right) (n = 4 different areas, photo of amoxicillin west ward with at least 100 cells per area). e Western blot analyses of BRCA1, RAD51, DNA damage markers and H3K9Ac in T47D cells overexpressing ctrl vs. BRCA1 ORF and treated with SOC for 4 hours. f Western blot analyses of DNA repair proteins, BRCA1, BRCA2, FEN1 and XRCC1 and G1/S transition marker, p-RB (S807/811) and p-PKA (Thr197) in T47D cells treated with SOC in a time-dependent manner. g Chromatin occupancy of PARP1 and HPF1 upon treatment of T47D cells with SOC therapies for 2 hours. Histone H3 and α-tubulin were used as the loading controls for nuclear and cytosol fractions, respectively, here and in all relevant figures. h Western blot analysis of ADP ribosylation (ADPR) in T47D cells treated with SOC for 1 hour. i PARP1 immunoprecipitation (IP) in SOC-treated T47D cells followed by immunoblotting for PARylation. j Western blot analysis of H3S10 ADPR, acetylated H3K9 (H3K9Ac), Histone H3 and cleaved PARP in T47D cells treated with SOC for 24 hours. k Relative EU incorporation in SOC-treated cells to show blockage of global transcription (n = 3). l Representative images of the EU staining (green) in SOC-treated cells from k. Scale bar = 200 µm. m Chromatin occupancy of PARP1 and HPF1 upon treatment of T47D shCtrl vs. shPARP1 cells with SOC therapies for 2 hours. n Western blot analysis of H3K9Ac in T47D shCtrl vs. shPARP1 cells treated with SOC for 4 hours. o Percentage growth inhibition in T47D cells with shPARP1 and treated with increasing doses of SOC for 5 days (n = 4). Actin is used as a loading control in in all Western blots unless stated otherwise. Data are presented as mean values ± standard deviation (SD). P-values were calculated with paired (o) or unpaired (d, k), two-tailed Student’s t test. n.s., not significant (P > 0.05). µM: micromolar, for all figures. Experiments in e-j, m are repeated twice with similar results. Source data for this figure are provided as a Source Data file. Credit: Nature Communications (2023). DOI: 10.1038/s41467-023-42736-y” width=”800″ height=”530″>
Researchers at MUSC Hollings Cancer Center believe that some drugs already approved by the FDA or currently in clinical trials could be repurposed for certain breast cancer patients whose cancer has become resistant to standard therapies.
Ozgur Sahin, Ph.D., a professor and SmartState Endowed Chair in the Department of Biochemistry and Molecular Biology, led the research, which was published Nov. 2 in Nature Communications.
The research started as an investigation into cancer resistance to the drug tamoxifen but expanded as the research questions led down new avenues, encompassing other hormone therapies and CDK4/6 inhibitors.
The result is a new explanation of why certain widely used therapies work—and by better understanding how these common therapies are actually functioning, researchers can formulate new therapies to respond when they stop working.
Christina Annunziata, M.D., Ph.D., senior vice president for extramural discovery science at the American Cancer Society, praised the work.
“The American Cancer Society is proud to support Dr. Sahin’s research into overcoming resistance to therapy in ER-positive breast cancer. This study underscores his novel approach to understanding molecular mechanisms of resistance underlying metastatic recurrence; as such, his research helps advance our mission to ensure that everyone has an opportunity to prevent, detect, treat and survive cancer,” she said.
Sahin focused on estrogen receptor positive, or ER-positive, breast cancer. ER-positive breast cancer, which accounts for about 75% of all breast cancer cases, uses estrogen to grow. Women with ER-positive breast cancer may use hormone therapy after surgery to prevent the cancer from returning or, if the cancer has already spread, they may use it to slow or stop its spread. CDK4/6 inhibitors, targeted therapies that stop cancer cells from multiplying, may be used at the same time as or after hormone therapy, depending on the specific diagnosis.
However, some patients may find that their cancer develops resistance to hormone therapy and CDK4/6 inhibitors.
“What we wanted to do in this study was to identify the initial question of how these therapies work and why they don’t work when patients develop resistance. And the third thing is—how we can make them work again?” Sahin said.
Cancer drugs are broadly divided into two categories based on how they act. Cytotoxic drugs kill cancer cells and cytostatic drugs slow or prevent cancer growth.
Hormone therapy has been categorized as cytostatic because it blocks estrogen receptors, but Sahin said his team found a new mechanism by which hormone therapy, also called endocrine therapy, is acting.
“What we show here is they are actually inducing DNA damage like chemotherapy agents,” he said. “This is so surprising and intriguing because we’ve found that these endocrine therapies and CDK4/6 inhibitors induce DNA damage with inhibition of homologous recombination, leading to toxic PARP trapping and cell death.” Recombination is a process by which pieces of DNA are broken and recombined to produce new combinations. PARP is a key protein involved in DNA repair.
“And since PARP is trapped on the chromatin, the transcription [the process of copying a gene’s instructions onto messenger RNA in order to build proteins] cannot happen. This is beyond the known blockage of estrogen receptor-dependent transcription by endocrine therapies,” he explained.
“We show that this is happening because when we give the standard-of-care therapies, they induce cyclic AMP,” added Ozge Saatci, a senior graduate student in the Sahin Lab and the first author of the paper. “These are second messengers in the cells. They are small molecules, and they have a lot of functions, but since these standards-of-care therapies lead to accumulation of cyclic AMP, so they generate reactive oxygen species, which leads to DNA damage.”
Sahin added that when cancer cells become resistant to hormone therapy, what is actually happening is that the cancer is becoming less dependent on estrogen to grow. Instead, it turns to other molecules like epidermal growth factor, or EGF.
Sahin’s team showed that targeting epidermal growth factor receptors (EGFR) or phosphodiesterase or using PARP inhibitors would overcome the cancer’s resistance to standard therapies.
“The most important thing here is that there are inhibitors for all those three which are either FDA-approved for different cancers, or they are in clinical trials for other non-cancer diseases,” Sahin said.
For example, a phosphodiesterase 4D inhibitor is in clinical trials for Fragile X Syndrome, a genetic disorder that causes developmental delays and intellectual disabilities, particularly in boys. Another clinical trial is testing PARP inhibitors in combination with endocrine and CDK 4/6 inhibitors. However, Sahin noted that the trial targets patients with BRCA mutations, and his team’s research indicates that the PARP inhibitors could also work for patients without BRCA mutations.
More information:
Ozge Saatci et al, Toxic PARP trapping upon cAMP-induced DNA damage reinstates the efficacy of endocrine therapy and CDK4/6 inhibitors in treatment-refractory ER+ breast cancer, Nature Communications (2023). DOI: 10.1038/s41467-023-42736-y
Journal information:
Nature Communications
Source: Read Full Article