Antioxidant Enzyme Systems
There are several enzyme systems that catalyze reactions to neutralize free radicals and reactive oxygen species. These enzymes include:
- superoxide dismutase
- glutathione peroxidise
- glutathione reductase
- catalases
These form the body’s endogenous defence mechanisms to help protect against free radical-induced cell damage. The antioxidant enzymes – glutathioneperoxidase, catalase, and superoxide dismutase (SOD) – metabolize oxidative toxic intermediates.
These enzymes also require co-factors such as selenium, iron, copper, zinc, and manganese for optimum catalytic activity. It has been suggested that an inadequate dietary intake of these trace minerals may compromise the effectiveness of these antioxidant defense mechanisms. The consumption and absorption of these important trace minerals may decrease with aging.
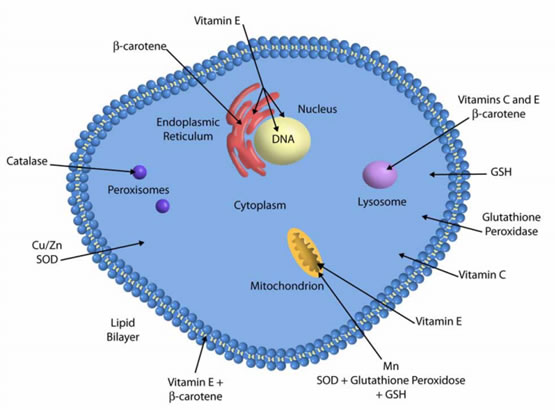
Glutathione enzymes and system
Glutathione, an important water-soluble antioxidant, is synthesized from the amino acids glycine, glutamate, and cysteine. Glutathione can directly neutralize ROS such as lipid peroxides, and also plays a major role in xenobiotic metabolism.
Xenobiotics are toxins that the body is exposed to. Exposure of the liver to xenobiotic substances means the body prepares itself by increasing detoxification enzymes, i.e., cytochrome P-450 mixed-function oxidase.
When an individual is exposed to high levels of xenobiotics, more glutathione is utilized for conjugation. Conjugation with Glutathioone renders the toxin neutral and makes it less available to serve as an antioxidant. Research suggests that glutathione and vitamin C work interactively to neutralize free radicals. These two also have a sparing effect upon each other.
The glutathione system includes glutathione, glutathione reductase, glutathione peroxidases and glutathione ''S''-transferases. Of these glutathione peroxidase is an enzyme containing four selenium-cofactors that catalyzes the breakdown of hydrogen peroxide and organic hydroperoxides. Glutathione ''S''-transferases show high activity with lipid peroxides. These enzymes are at particularly high levels in the liver.
Lipoic acid
This is another important endogenous antioxidant. It is categorized as “thiol” or “biothiol”. These are sulfur-containing molecules that catalyze the oxidative decarboxylation of alpha-keto acids, such as pyruvate and alphaketoglutarate, in the Krebs cycle.
Lipoic acid and its reduced form, dihydrolipoic acid (DHLA), neutralize the free radicals in both lipid and aqueous domains and as such has been called a “universal antioxidant.”
Superoxide dismutase
Superoxide dismutases (SODs) are a class of enzymes that catalyse the breakdown of the superoxide anion into oxygen and hydrogen peroxide. These enzymes are present in almost all aerobic cells and in extracellular fluids.
SODs contain metal ion cofactors that, depending on the isozyme, can be copper, zinc, manganese or iron. For example, in humans copper/zinc SOD is present in the cytosol, while manganese SOD is present in the mitochondrion. The mitochondrial SOD is most biologically important of these three.
In plants, SOD isozymes are present in the cytosol and mitochondria. There is also an iron SOD found in chloroplasts.
Catalases
Catalases are enzymes that catalyse the conversion of hydrogen peroxide to water and oxygen, using either an iron or manganese cofactor. This is found in peroxisomes in most eukaryotic cells. Its only substrate is hydrogen peroxide. It follows a ping-pong mechanism.
Here, its cofactor is oxidised by one molecule of hydrogen peroxide and then regenerated by transferring the bound oxygen to a second molecule of substrate.
Peroxiredoxins
There are peroxidases that catalyze the reduction of hydrogen peroxide, organic hydroperoxides, as well as peroxynitrite. These may be of three basic types – typical 2-cysteine peroxiredoxins; atypical 2-cysteine peroxiredoxins; and 1-cysteine peroxiredoxins. Peroxiredoxins seem to be important in antioxidant metabolism.
Sources
- http://acudoc.com/Antioxidants.PDF
- http://ocw.jhsph.edu/courses/humannutrition/PDFs/Lecture8.pdf
- http://www.womenfirst.net/pdf/ADA/ADA_Antioxidants.pdf
- http://class.fst.ohio-state.edu/fst821/Lect/AA.pdf
- http://www.medlabs.com/Downloads/Antiox_acti_.pdf
- http://www.terry-oberley.net/59.pdf
- http://hera.ugr.es/doi/16518494.pdf
Further Reading
- All Antioxidant Content
- What are Antioxidants
- Antioxidant: The Oxidative Challenge In Biology
- Antioxidant Metabolites
- Antioxidant: Pro-Oxidant Activities
Last Updated: Feb 26, 2019

Written by
Dr. Ananya Mandal
Dr. Ananya Mandal is a doctor by profession, lecturer by vocation and a medical writer by passion. She specialized in Clinical Pharmacology after her bachelor's (MBBS). For her, health communication is not just writing complicated reviews for professionals but making medical knowledge understandable and available to the general public as well.
Source: Read Full Article
