Diabetic foot wounds kill millions, but high-tech solutions and teamwork are making a difference
What if someone told you that there’s a disease you could catch where you couldn’t feel any symptoms coming on? And that this occurs every 1.2 seconds somewhere in the world?
What if you were stricken with this disease then there would be a 5% chance you’d lose a limb within a year and a 50-70% chance you’d be dead in five years? What if you were told that this problem cost more than the five most expensive cancers in the U.S. but far less than one one-thousandth of comparative federal and private funding is spent on attacking it?
Ladies and gentlemen, please allow me to introduce you to the humble diabetic foot ulcer. While the problem may strike at the end of the body, far away from the heart or the brain, its effects are far-reaching.
I have spent my career treating and researching the lower extremity complications of diabetes. Based on my research and experience, I believe our society could eliminate immeasurable suffering if we collectively paid more attention to this problem.
How do diabetic foot ulcers occur?
OK, I know this isn’t a sexy topic. Foot wounds are ugly. Many people who have them are poor. But bear with me. They are a reality for far too many Americans and people across the globe. The ages of these patients are “bimodal,” in that there is one population of people who are old and getting older. Conversely, with more and more people being diagnosed with Type 2 diabetes earlier, there is a population that is younger than ever being afflicted with wounds, infections and amputation. Ignoring the problem is an example of ignoring the needs of a silent and vulnerable population.
About 31 million people in the U.S. have diabetes, and about half a billion worldwide.
Diabetic foot ulcers develop because people with diabetes slowly lose the “gift of pain.” Over many years, people with diabetes lose feeling in their extremities. This occurs first—and generally most profoundly—in their feet.
Once this occurs, people with diabetes might wear a hole in their foot, just as you or I might wear a hole in a sock or shoe. This “hole” is called a diabetic foot ulcer.
About half the time, the ulcer will become infected. This increases the risk of further tissue damage and, in the face of frequent vascular disease, high-level amputation. Often all of this occurs with few, if any, symptoms until it is too late.
Solutions and hope
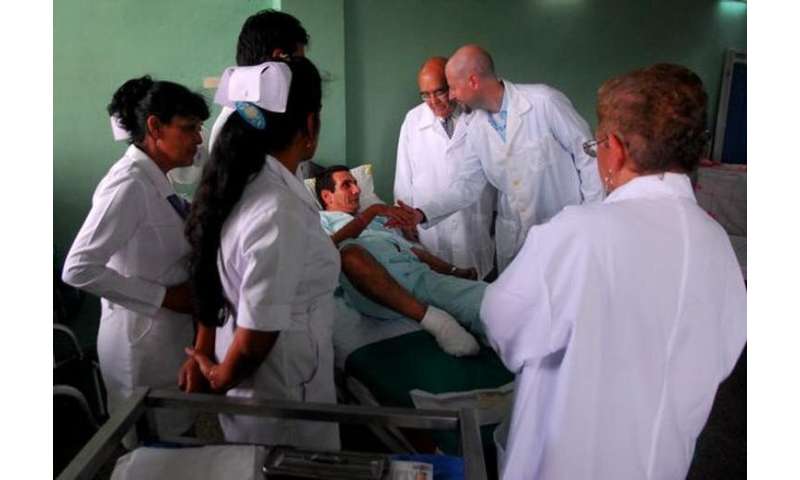
There is also good news. Studies have suggested that high-level amputations seem to decrease when interdisciplinary care is in place, regardless of the country.
Interdisciplinary teams consist of podiatric and vascular surgeons, the so-called “Toe and Flow” model. The concept is simple; these two specialists, can manage a great deal of the medical, surgical and biomechanical aspects of healing and aftercare.
When we add core physical therapy to this, then the threesome (what we in the field call “Toe, Flow and Go”) is really quite formidable. For example, our clinics at the University of Southern California and Rancho Los Amigos in Los Angeles have active participation from more than eight specialists ranging from plastic surgery to prosthetics/orthotics, to occupational therapy to nutrition to general practice to infectious disease to diabetology to nurse case management.
Truly, it takes a village to preserve a limb.
Smart boots, high-tech vacuums and sheets of stems cells
It has long been said in wound care that “it’s not what one puts on a wound that heals it, but what one takes off.” That maxim is absolutely true in the diabetic foot. Protection of the wound is key.
The gold standard for protecting the wound has been, believe it or not, to put the patient into a special cast. This device works so well because it protects the foot in a process known as offloading, or taking the burden off the foot. By its design, this cast is not easy to remove.
While this has been my personal favorite device to heal these foot wounds, patients don’t like it and most doctors don’t, either. In fact, fewer than 2% of centers in the country use this as their primary means of offloading. Reasons for this include fear of putting an open wound into a cast (even though the data largely refute this), the time required to apply and remove it and patients being miserable in a hot and heavy device.
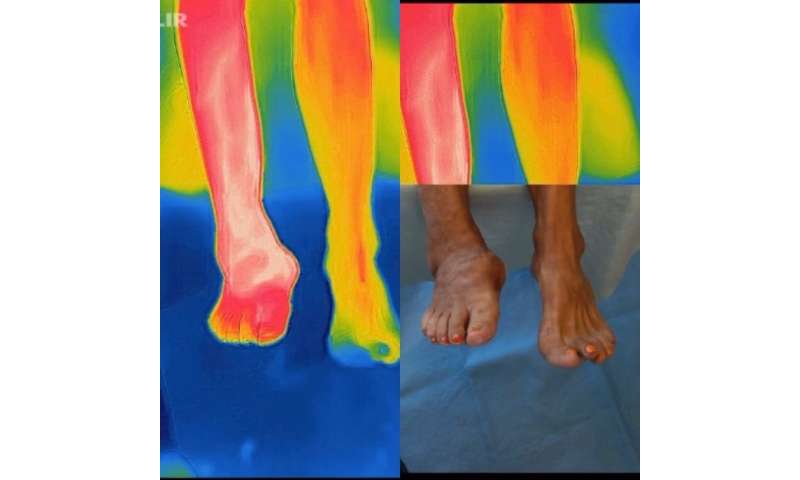
Very recently, tech company offshoots have begun to partner with prosthetic/orthotic companies to create next-gen devices that can coax patients into wearing their protective device rather than forcing it upon them. They are using phone calls and a smartwatch.
After focusing on offloading pressure, the next question is what can be done to heal the wound.
Technologies ranging from fancy vacuums, to donated placental tissue, to repurposing blood cells into a dressing to topical oxygen systems have shown recent promise. Active research is being conducted with stem cell sheets consisting of specialized cells seeded on a clear sheet, spread-on skin, and gene therapy.
Remission
As challenging as healing the wound heals, the real challenge is what’s next. Following healing, 40% of foot wounds will recur in one year, about two-thirds at three years, and nearly three out of four at five years.
At USC, along with colleagues in the National Health Service in the U.K., we have developed “remission clinics” designed to extend and promote an active life for this high-risk patient population.
This has also been combined with things like smart insoles, socks and home-based bathmats that can identify wounds before they occur. These technologies will likely initially be “subscription-based” but may expand beyond that.
Source: Read Full Article
