Clinicians may soon be able to tell COVID-19 from seasonal flu with fast turnaround

For those who have made a trip to a doctor or clinic during the oncoming flu season, the most nagging concern is whether their symptoms are indicative of the garden-variety bug that is making the rounds in the workplace or a potential COVID-19 infection. As researchers scramble to treat this virus—and its inevitable variants and mutations—one of the biggest challenges has been quickly and accurately diagnosing the source of the illness so that the proper treatment protocols are administered, and in time to decrease the odds of potentially dangerous complications.
Led by Dr. Z. Hugh Fan, Ph.D., professor at the Herbert Wertheim College of Engineering’s Department of Mechanical and Aerospace Engineering, and Dr. John Lednicky, Research Professor at the College of Public Health and Health Professions’ Department of Environmental and Global Health, an interdisciplinary team at the University of Florida has developed a game-changing diagnostic test for SARS-CoV-2 that is fast, reliable, low-cost and capable of differentiating between COVID-19 and influenza.
Dr. Fan and his team demonstrated the ability to detect two viruses in 50 minutes without using bulky or sophisticated laboratory equipment or power supply, and with detection sensitivity that is on par with that of the gold-standard RT-PCR (reverse transcription polymerase chain reaction) assay, which is the test recommended by the World Health Organization and the Centers for Disease Control and Prevention. The effort is supported by the National Institutes of Health.
“In patient-care terms, this innovative sample-to-answer platform is an important upgrade from the existing RT-PCR standard on several levels,” Dr. Fan said. “With regard to diagnosis, the turnaround time is less than an hour at the testing spot compared to one to two days from an offsite testing lab.”
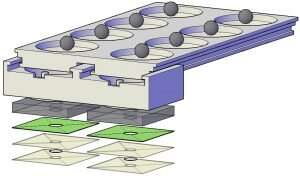
The UF-engineered device was recently published for peer review in ACS Sensors, the flagship journal of the American Chemical Society, focusing on sensor science.
RT-LAMP (loop-mediated isothermal amplification) is an assay used for viral RNA detection. The methodology for RT-LAMP was based on the mechanism behind auto-cycling strand displacement DNA synthesis. A polymerase carries out the reaction, and the polymerase has high strand displacement activity. There are also two pairs of primers used, one pair of inner and another of outer primers. These primers are specially designed for the reaction. RT-LAMP achieves high specificity due to the target sequences. Unlike other technologies, RT-LAMP recognizes the target sequence using six independent sequences at the start, giving it high specificity. Primer recognition of the target genome leads to a strong colorimetric reaction, which allows for detection without highly specialized or costly instrumentation.
“RT-LAMP technology has numerous advantages over standard RT-PCR,” Dr. Lednicky said. “The cost of reagents is low compared to an equivalent RT-PCR reaction, results are obtained more quickly, and equipment needs for RT-LAMP are low, which is a major advantage for users in resource-strapped countries or for use in doctor’s offices and clinics. Also, packaged RT-LAMP reactions in a kit format take up little storage space and can be freeze-dried, alleviating the need for freezer storage.”
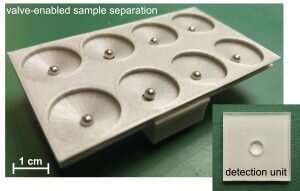
The specimen—swab, urine, saliva, blood, depending on the test—is taken, transferring a determined amount into the first chamber of the sample work-up device. Sequentially, the specimen is subjected to specific fluids that extract DNA or RNA from the pathogen, purifying the extracted material and collecting it on a detachable filter unit. RT-LAMP chemicals are added into the detachable unit, the chamber with the RT-LAMP fluids is sealed with tape and the final unit is detached and placed into a microchip-controlled “coffee mug,” which is pre-heated to a specific temperature. After a selected incubation period, the results are determined, evidenced by a color change that is visually detected or that can also be detected/measured through its level of fluorescence.
Using disposable-format single-use units, the device can be used by nurses, doctors, and others without the need for advanced training. The results, positive or negative, can be determined visually or with the aid of simple technology like a flashlight. The results can be recorded by using an iPhone or similar camera-equipped mobile device to capture the image, where it is sent to a remote site for confirmation of the data.
“Bottom line, the device allows for quicker analysis of genetic material than traditional RT-PCR and has been successfully used in the detection of the SARS-CoV-2 virus,” Dr. Lednicky said. “It’s simple, cheap, and does not require advanced technical skills or expensive equipment. Most importantly, it’s very sensitive, yielding detection sensitivities, in some cases, 10 times more sensitive than standard RT-PCR assays.”

The simultaneous test of both SARS-CoV-2 and influenza viruses isn’t presently available at point-of-care (POC), but its variations are available in test labs, and would likely be considered as a “CLIA-waived” device, which includes home tests approved by the FDA. “The device could also be used for environmental studies (SARS-CoV-2 in air, sewage or other matrices), and can be affixed/mated with other detection devices such as air samplers,” Dr. Fan said.
The development of this LAMP-based device comes on the heels of progressive advancements by UF researchers in the early detection and identification of dangerous pathogens. Three years ago, with funding from the State of Florida, Dr. Fan and Dr. Lednicky led a research team that developed a rapid, cost-effective miniaturized device for the detection of the mosquito-borne Zika virus, which presents no symptoms in up to 80 percent of those infected. The point-of-care testing platform can be used for screening asymptomatic patients in the field, mitigating misdiagnosis and helping to prevent further transmission.
Source: Read Full Article
