New intracellular ‘smoke detector’ discovered
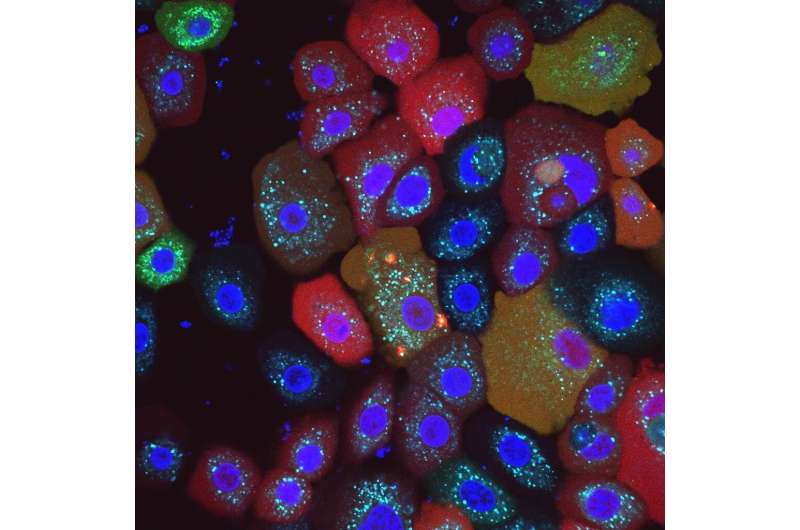
Researchers at the Universities of Bonn and Singapore have discovered a new intracellular “smoke detector.” The sensor warns of damage to the mitochondria—the microscopic power plants that supply the cell with energy. If it does not function properly, chronic skin diseases can result.
The sensor may also be important for unimpaired heart and bowel function.
The results have now been published in the journal Nature Immunology.
Every cell in the body has numerous sensors that monitor its function. Some sound the alarm after a virus attack, for instance; others kick in when any kind of damage threatens the cell’s survival. “We have now discovered that a molecule called NLRP10 also acts as a sensor,” explains Prof. Dr. Eicke Latz, head of the Institute of Innate Immunity at the University Hospital Bonn. “This was completely unknown until now.”
Figuratively speaking, NLRP10 detects when the mitochondria in the cell start to smoke due to some malfunction. These are the microscopic power plants that provide the energy for cellular functions. As soon as an NLRP10 sensor detects damage to mitochondria, it sets off a complicated process.
This creates a so-called inflammasome, a complex molecular machine. Its activity ultimately causes the cell to perish and be disposed of by summoned immune cells.
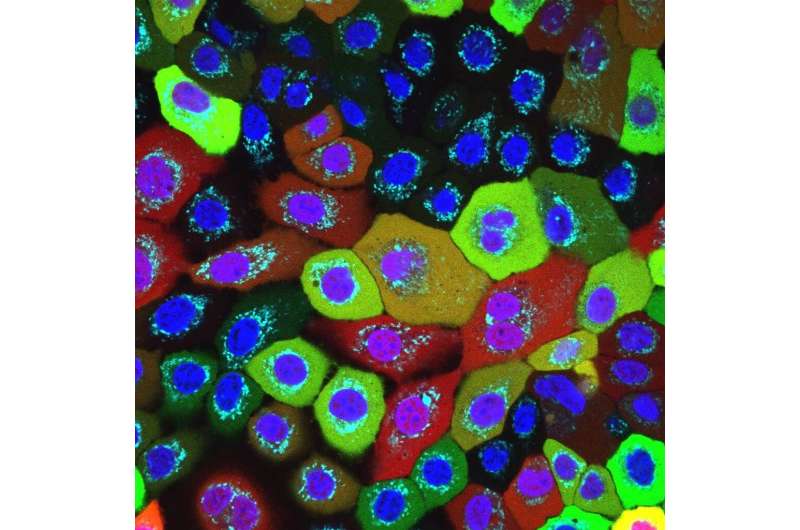
Fire alarm prevents long-lasting smoldering fire
“This process is hugely important,” explains Latz, who is also the spokesperson for the Cluster of Excellence ImmunoSensation2 and a member of the Transdisciplinary Research Area “Life and Health” at the University of Bonn. This is because the inflammasome ensures that the fire is stamped out straight away, which prevents a prolonged smoldering fire that would damage other parts of the tissue.
“Disruption of this mechanism can result in chronic inflammation,” the researcher emphasizes. “Killing cells with mitochondrial defects may sound drastic. Ultimately, however, this step prevents more serious consequences.”
Not all cells in the body have an NLRP10 sensor. The “fire detector” occurs primarily in the outermost skin layer, the stratum granulosum. The skin is directly exposed to environmental stimuli such as UV radiation, but also pathogens. This could potentially result in accumulated damage. The mechanism ensures that affected cells are effectively disposed of. “If a mutation causes the NLRP10 sensor to malfunction, this can result in a chronic skin inflammation called atopic dermatitis,” explains Dr. Tomasz Próchnicki, who performed an important part of the experiments for his doctorate in Latz’s research group.
Large quantities of NLRP10 are also found in the intestinal wall cells. These also have regular contact with pathogens and potentially harmful substances.
Another organ in which the sensor can be detected is the heart. It is particularly dependent on a well-functioning energy supply. This may make it especially important to quickly kill and replace cells with defective mitochondria.
The study may potentially also open up new therapeutic perspectives. “It is conceivable to specifically modulate the NLRP10 sensor using certain substances in order to stimulate the formation of inflammasomes,” Latz explains. “This approach might enable chronic skin diseases to be better controlled.”
More information:
Eicke Latz, Mitochondrial damage activates the NLRP10 inflammasome, Nature Immunology (2023). DOI: 10.1038/s41590-023-01451-y. www.nature.com/articles/s41590-023-01451-y
Journal information:
Nature Immunology
Source: Read Full Article
