Prior MMR or Tdap vaccination may reduce risk of severe COVID-19
Researchers in the United States have shown that prior Measles-Mumps-Rubella (MMR) or Tetanus-Diphtheria-pertussis (Tdap) vaccination may reduce the risk of severe outcomes in patients with coronavirus disease 2019 (COVID-19).
The team provided evidence that infection with the COVID-19 causative agent – severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – reactivates memory T cells previously generated by MMR and Tdap vaccination and that this may reduce the severity of COVID-19.
"Our findings provide definitive cellular and molecular evidence that heterologous adaptive immunity exists between SARS-CoV-2 and antigens present in Tdap and MMR vaccines," say the researchers from Brigham and Women's Hospital & Harvard Medical School in Boston, Massachusetts, the University of California Davis, and the Cleveland Clinic in Ohio.
"Heterologous adaptive immunity" refers to an immune response to one pathogen that is mediated by the memory T cells previously generated against a different pathogen, either through infection or vaccination.
"Heterologous immunity can variously alter disease outcomes by providing enhanced immunity or by exacerbating immunopathology or lessening viral control," says Tanya Mayadas and colleagues.
In an extensive analysis of more than 73,000 COVID-19 patients, the team found that severe disease outcomes were reduced among individuals previously vaccinated against MMR or Tdap.
The researchers say the findings may have implications for the development of vaccines against future novel pathogens since a vaccine's efficacy may correlate with its ability to harness pre-existing memory T cells generated by prior infection or vaccination.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.
.jpg)
T cells are critical to controlling viral infection
T cells are critical in the early control of acute viral infection and for stimulating B cells to produce protective antibodies against viral antigens.
The expansion of T cells following productive immunity typically generates a memory T cell population that can persist for decades and rapidly respond to reinfection.
A major goal in vaccine development is, therefore, the induction of strong and durable T-cell memory.
Studies of SARS-CoV-2-specific T-cell responses have suggested that key viral targets include the spike protein (that mediates the initial stage of host cell infection), the nucleocapsid protein (that packages viral RNA into new virions) and the envelope protein (needed for replication).
Interestingly, several studies have shown that memory T cells that are specific to coronaviruses related to SARS-CoV-2 – including those that cause the common cold – cross-react with SARS-CoV-2 antigens.
"Moreover, profiling of the TCR [T cell receptor] repertoire of T cells isolated from unexposed and SARS-CoV-2 convalescent patients and expanded in vitro with predicted immunodominant viral peptides show clonal expansion of T cells with TCR sequences recognizing peptides from other viruses including human cytomegalovirus and influenza A," says Mayadas and colleagues.
However, "the impact of these pre-existing, cross-reactive memory T cells on disease outcomes are largely unknown," writes the team.
What did the researchers do?
The researchers used an assay for antigen-specific T cell responses to determine whether SARS-CoV-2 protein-specific T cells from convalescent individuals or recipients of COVID-19 vaccines cross-reacted with antigens present in MMR and Tdap vaccines.
The MMR and Tdap vaccines are known to be highly effective at eliciting long-lasting protective immunity and therefore T and B cell memory responses, explains the team.
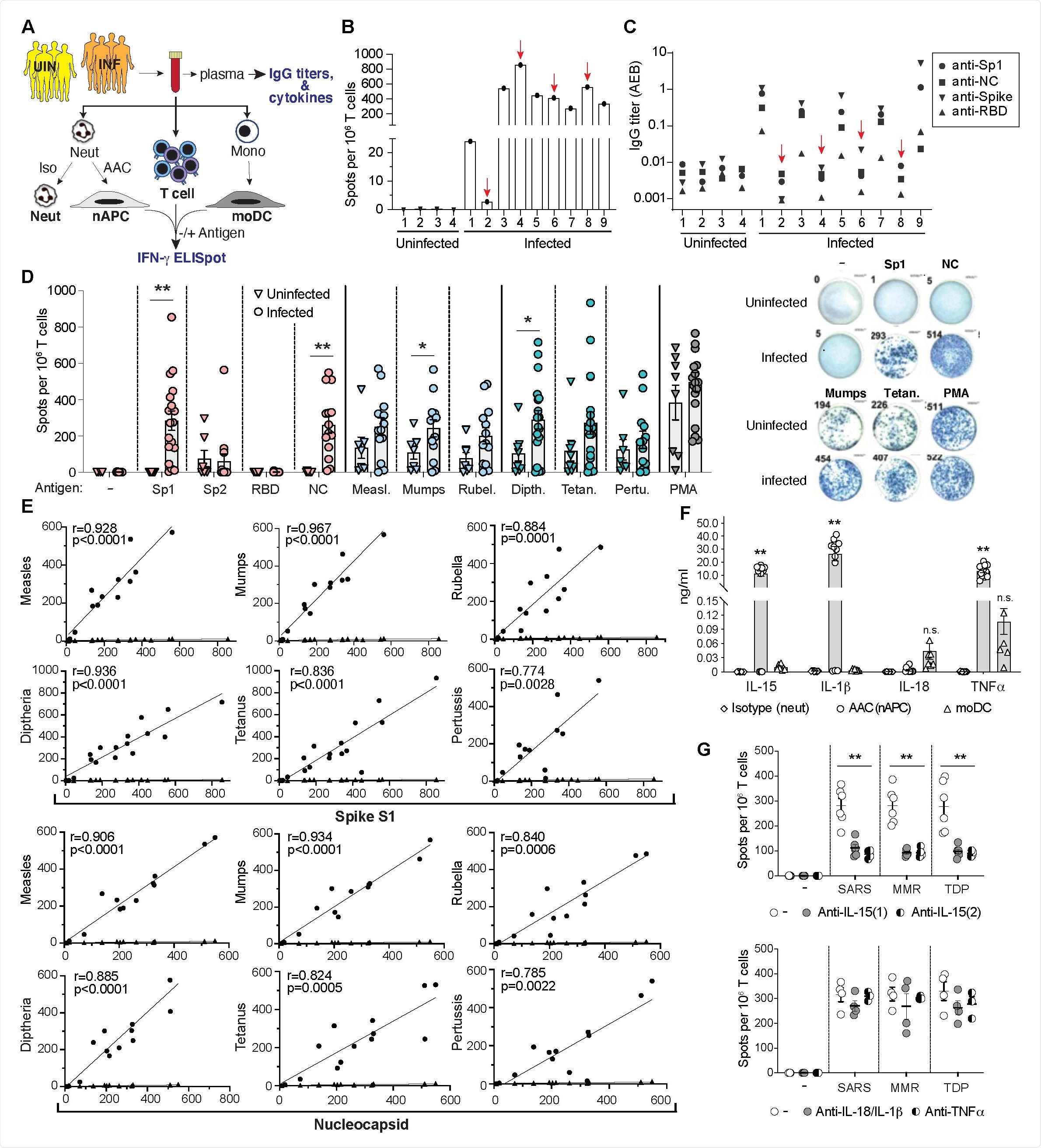
The researchers identified a strong correlation between responses to the SARS-CoV-2 spike and nucleocapsid proteins and responses to MMR and Tdap vaccine proteins in both convalescent individuals and those who had been immunized with COVID-19 vaccines.
The researchers say the findings suggest that the MMR and Tdap reactive memory T cells will enhance immunity to the SARS-CoV-2 spike variants that may emerge as population immunity continues to expand.
"In uninfected individuals immunized with the SARS-CoV-2 vaccine, the markedly enhanced T cell responses to MMR and Tdap antigens compared to the same evaluated prior to vaccination suggests that protective heterologous T cell immunity in vaccinated individuals may be induced by MMR and Tdap vaccine antigens," they write.
Could MMR or Tdap vaccination alleviate severe disease in COVID-19 patients?
Next, the team conducted a propensity-weighted analysis of 73,582 COVID-19 patients adjusted for multiple patient characteristics to determine whether disease severity was attenuated in individuals with prior MMR or Tdap vaccination.
This revealed that severe outcomes (hospitalization, transfer to intensive care or death) were reduced in MMR- and Tdap- vaccinated individuals by 38-32% and 23-20%, respectively.
What are the implications for vaccine development?
"Our studies provide evidence of broad cross-reactivity between T cells responsive to SARS-CoV-2, MMR and Tdap antigens in humans," writes Beck and colleagues.
The researchers say the prevalence of heterologous immunity observed here may have implications for vaccine development against future novel pathogens:
"We posit that intentional MMR or Tdap vaccine-induced heterologous immunity to SARS-CoV-2 could enhance the effectiveness of SARS-CoV-2 vaccines by generating an expanded population of SARS-CoV-2-specific memory T cells (and, in turn, B cells) that respond vigorously to the vaccines and, in countries where SARS-CoV-2 vaccines are not yet available, provide protection from severe disease."
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Mayadas T, et al. Protective heterologous T cell immunity in COVID-19 induced by MMR and Tdap vaccine antigens. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.05.03.441323, https://www.biorxiv.org/content/10.1101/2021.05.03.441323v1
Posted in: Medical Research News | Disease/Infection News
Tags: Antibodies, Antigen, Assay, Blood, CD3, Cell, Cold, Common Cold, Coronavirus, Coronavirus Disease COVID-19, Cytokine, Cytokines, Cytomegalovirus, Diphtheria, Efficacy, Frequency, Hospital, Immune Response, in vitro, Influenza, Intensive Care, Measles, Medical School, MMR, Monocyte, Mumps, Neutrophils, Pathogen, Peptides, Pertussis, Protein, Receptor, Research, Respiratory, RNA, Rubella, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, T-Cell, Tetanus, Vaccine

Written by
Sally Robertson
Sally first developed an interest in medical communications when she took on the role of Journal Development Editor for BioMed Central (BMC), after having graduated with a degree in biomedical science from Greenwich University.
Source: Read Full Article
