Researchers identify a crucial gene that triggers congenital muscular dystrophy
For the targeted treatment of a disease, information is needed about the course of the disease at molecular and cellular level. This is also true for the group of muscle diseases that include congenital muscular dystrophies (CMD). Bielefeld scientists have been able to identify a crucial gene that triggers CMD when it ceases to function. The principal collaborator is the research group of Carsten G. Bönnemann, M.D., of the National Institutes of Health in the U.S.; numerous other international partners were involved in the research project. The collaboration is financed by third-party funds from the German Research Foundation (DFG). The results were published in the EMBO Molecular Medicine journal on 15 November 2021.
Congenital muscular dystrophies encompass a group of diseases whose main feature is the degeneration of muscles: progressive muscle weakness and muscle wasting. Symptoms appear at birth or early during infancy. Children’s motor development—such as head control, sitting freely or crawling—is delayed or not completed. In some cases, muscle weakness is so severe that no movement against gravity is possible. Depending on the type of CMD, muscle weakness also affects organs such as the lungs or the heart and leads to a shortened life expectancy. In some types, the central nervous system is affected.
The various types of CMD have different causes. The disease can be triggered by rare variants in genes. Scientists distinguish between variants of the disease according to which gene sequences are affected and what the corresponding consequences are at molecular and cellular level.
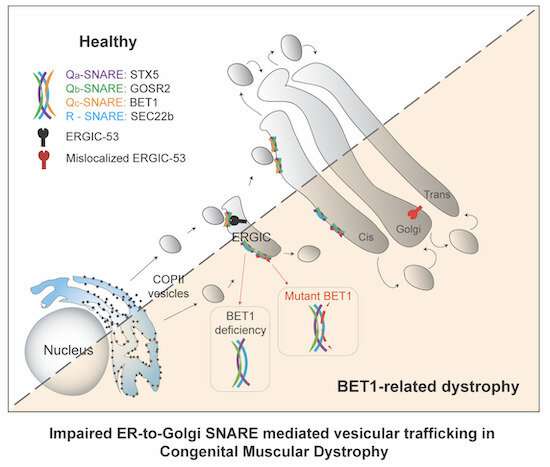
The authors have now been able to identify a ‘new’ gene as causative: the BET1 gene. It plays an important role in transport within the cell. “There is a lively exchange in our cells,” says PD [senior lecturer] Dr. Michael Schwake, supervisor of the collaborative research. “Proteins that function as messengers or building blocks, for example, have to be moved from where they are produced to where they are needed. There is a separate transport system in the cells for this purpose: the vesicles.Imagine it as being like trucks shuttling cargo. Vesicles are self-contained structures—little bubbles—that capture materials, transport and deliver them to their destinations.”
The BET1 gene is involved in this cellular transport system. “The vesicles are composed of different building blocks. One of them is encoded by the BET1 gene that is produced according to its genetic instructions,” says the researcher. This process is impaired in the CMD patients in the study. “We have been able to show that the BET1 gene loses its transport mechanism function when a certain mutation occurs. This has enabled us to identify what is known as a loss-of-function mutation for the BET1 gene that triggers CMD. Until now, this gene has not been linked to disease in humans.”
CMD is often diagnosed by genetic testing, but not all relevant gene sequences have yet been identified. At present, a differential diagnosis is often made—physicians rule out other conditions that cause similar symptoms. “A lot of patients suffer from CMD without having received a genetic diagnosis,” says Schwake. “This is partly because we still know too little about the genetic causes and their molecular consequences. Yet, they can form the basis for a clear, individual diagnosis and targeted treatment.”
A person’s genetic disposition—in other words, the hereditary characteristics they were born with—can be decisive and may be the reason why a drug does not necessarily work for particular patients: “The genetic cause of CMD is very complex, but it points to which cell mechanism is affected. The mechanism varies greatly between the different types of CMD. A targeted drug acts on a defined mechanism, which is why the same drug cannot be equally effective for everyone,” explains Schwake.
At the same time, the scientists’ pure research provides a perspective. “Hopefully, this knowledge can be used to develop new drugs. In the long term, it could then be possible to see where therapy has to start in the cell for a particular person. That’s the basic idea of personalized medicine: drugs that exactly match the genetic disposition of the individual person.
Source: Read Full Article
