exelon energy salaries
In a recent study posted to the medRxiv* preprint server, researchers investigated whether the upsurge in respiratory syncytial virus (RSV) infections during the autumn season of 2022 occurred due to the emergence of a novel, highly transmissible RSV strain or whether multiple pre-existing RSV strains were responsible for the increase in RSV case counts during the period.
In 2022, paroxetine 20 mg for premature ejaculation an unusually untimely and extreme upsurge in RSV infections was observed in the United States (U.S.), which increased the health burden of RSV infections for hospitals and healthcare centers. However, despite the increase in medical visits and hospital admissions due to RSV infections, the causative factors of the upsurge are unknown.
 Study: The 2022 RSV surge was driven by multiple viral lineages. Image Credit: B-th / Shutterstock
Study: The 2022 RSV surge was driven by multiple viral lineages. Image Credit: B-th / Shutterstock
About the study
In the present study, researchers investigated whether the emergence of a novel and highly virulent strain of RSV resulted in the upsurge in RSV infections witnessed in the U.S. during autumn 2022.
Whole genome sequencing (WGS) analysis was performed for RSV-positive upper respiratory tract specimens (n=105) obtained from individuals with symptomatic RSV infections clinically diagnosed at the MGH (Massachusetts general hospital) or its affiliated outpatient settings in Greater Boston. RSV infections were diagnosed based on polymerase chain reaction (PCR) analysis reports.
In addition, phylogenetic analysis was performed, and a maximum likelihood tree was constructed to identify genetic clades. To assess the epidemiological and genomic trends of the 2022 RSV upsurge, the number of (PCR)-positive test reports for RSV documented by the US CDC (Centers for Disease Control and Prevention) in Massachusetts and the number of RSV-positive test reports documented at the Massachusetts General Hospital were assessed.
RSV hospital admission rates reported in CDC’s RSV hospitalization surveillance network (RSV-NET) and MGH RSV cases for the period between 2017 and 2022 were compared. RSV genomes and raw sequence reads were submitted to Genbank for Bayesian analysis. Explosion plots of RSV-A and RSV-B strains in circulation during the autumn of 2022 with the inferred tMRCA (times to most recent common ancestor) values and associated confidence intervals (C.I.) for each strain were assessed.
Results
WGS analysis of 105 specimens generated 77 genomes of RSV, of which 23 genomes were partial (greater than 5.0% coverage), and 54 genomes were near-complete (with greater than 80.0% coverage), obtained from specimens with high viral loads [i.e., with cycle threshold (Ct) values below 30.0].
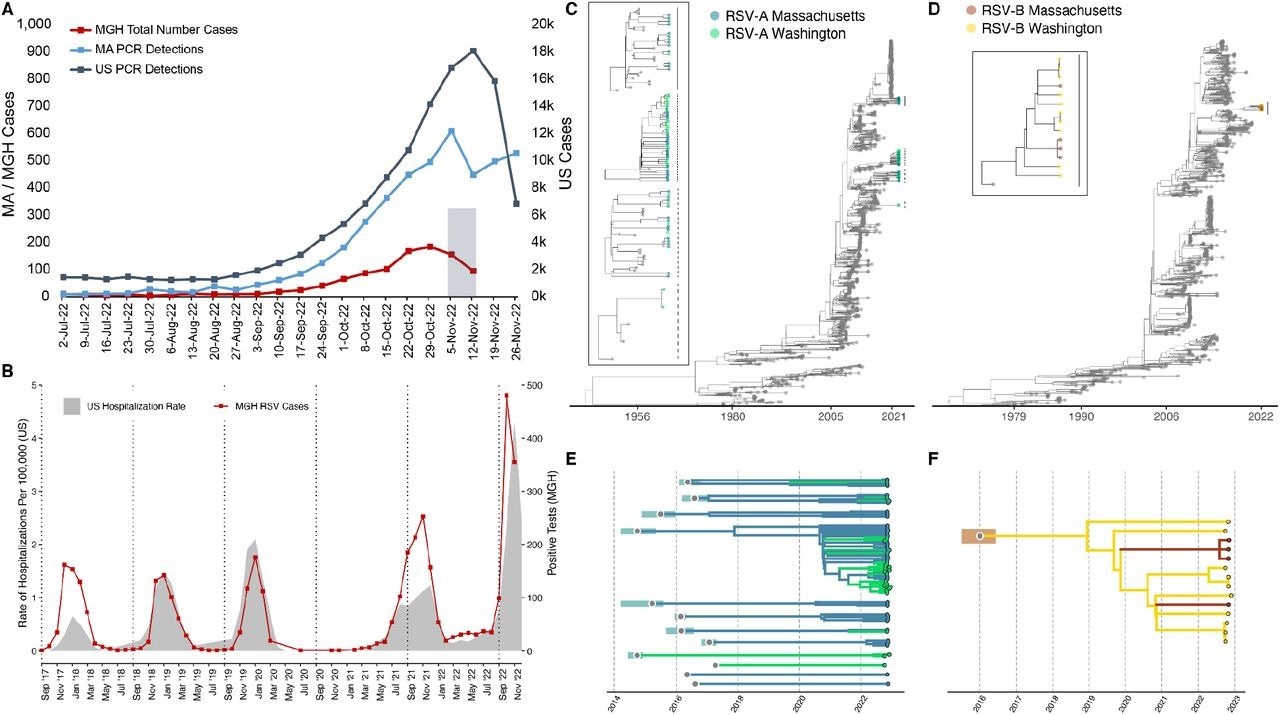
Epidemiological and genomic trends of the 2022 RSV surge. A) The number of PCR positive tests for RSV reported by the CDC in MA (blue, left axis) and the US (slate gray, right axis), along with the number of RSV positive tests conducted at MGH (red, left axis). The 105 sequenced samples were drawn from the gray Nov 2 – Nov 15 window. B) RSV hospitalization rates for CDC’s RSV-NET (shaded gray) and MGH RSV cases (red) for 2017 – 2022. (Pearson r = 0.82; p < 0.0001 via permutation). C) Maximum likelihood tree of all RSV-A genomes (N=1,267) (MA tips in blue, WA tips in green, others in gray). The tMRCA for 2022 RSV-A genomes was no later than 2008 (ML CI: 2008-04, 2008-09). In the box are zoomed in plots of the clades containing MA and WA with lines corresponding to clades on the tree. D) Maximum likelihood tree of all RSV-B genomes (N=944) (MA tips in orange, WA tips in yellow, others in gray). The tMCRCA for 2022 RSV-B genomes was approximately 2016 (ML CI: 2015-08, 2016-08). In the box are zoomed in plots of the clades containing MA and WA with lines corresponding to clades on the tree. Explosion plot of E) RSV-A and F) RSV-B lineages circulating in autumn 2022 with the inferred tMRCA (gray dots) and associated confidence intervals (shaded regions) for each lineage.
Genetics & Genomics eBook

In addition, the sequenced data showed nine and one co-infections with respiratory viral organisms such as enterovirus or rhinovirus and metapneumovirus, respectively. Phylogenetic analysis findings indicated that the upsurge occurred due to multiple RSV-A strains (70 out of 77 genomes, 91.0%) and strains of RSV-B (seven out of 77 genomes, 9.0%).
The near-complete RSV genomes showed the presence of genotypes GA2.3.5 and GB5.0.5a representing RSV-A and RSV-B, respectively. The genomes of RSV-A identified were of ≥10 different RSV strains, each having tMRCA in the period from 2014 to 2017, and the combined tMRCA for RSV-A genomes was up to 2009. The findings indicated that the genomes emerged much earlier than the SARS-CoV-2 pandemic.
Likewise, the tMRCA for genomes of RSV-B was in the period from 2016 to 2019. Currently, 39 other genomes of RSV were identified from the RSV upsurge in 2022 in the United States, originating from Washington. The genomes of RSV-A that originated from Washington were found to belong to six RSV strains, four of which also comprised Massachusetts genomes.
The genomic divergence exhibited by the 2022 respiratory syncytial virus type A genomic strains and 2022 respiratory syncytial virus type B genomic strains was concordant with the predicted clocking rates derived from the phylogenetic tree of 11 annual substitutions and 12 annual substitutions. The finding contrasted the rapid evolution of increasingly virulent SARS-CoV-2 variants of concern (VOCs) such as the Alpha VOC, Delta VOC, and the Omicron VOC.
Overall, the study findings showed that the upsurge in RSV infections occurring in the U.S. in the autumn season of 2022 occurred due to several pre-existing RSV strains, many of which were common between different geographical areas. The polyphyletic RSV genomes sequenced during 2022 do not indicate the rise of a highly virulent RSV strain as the reason for the upsurge. Further research must be conducted to evaluate the impact of factors that affect RSV spread and virulence, such as altered immunity levels with changes in RSV infection kinetics during the SARS-CoV-2 pandemic, to improve understanding of RSV epidemiology and improve global preparedness.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- The 2022 RSV surge was driven by multiple viral lineages. Gordon Adams, B.A. et al. medRxiv preprint 2023, DOI: https://doi.org/10.1101/2023.01.04.23284195, https://www.medrxiv.org/content/10.1101/2023.01.04.23284195v1
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: CT, Enterovirus, Epidemiology, Evolution, Genetic, Genome, Genomic, Healthcare, Hospital, immunity, Omicron, Pandemic, Polymerase, Polymerase Chain Reaction, Research, Respiratory, Respiratory Syncytial Virus, Rhinovirus, SARS, SARS-CoV-2, Virus, Whole Genome Sequencing

Written by
Pooja Toshniwal Paharia
Dr. based clinical-radiological diagnosis and management of oral lesions and conditions and associated maxillofacial disorders.
Source: Read Full Article
