phentermine brand name in pakistan
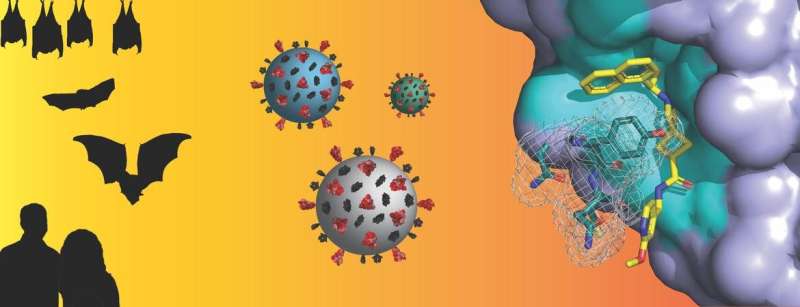
Both SARS-CoV-1, the virus that caused the 2003 outbreak of Severe Acute Respiratory Syndrome (SARS), and SARS-CoV-2, which causes COVID-19, originate from a group of betacoronaviruses known as “subgroup 2b.” Coronaviruses from this subgroup have been highlighted as having significant potential to cross from animal hosts to humans with deleterious consequences, COVID-19 being the most recent one.
A coronavirus enzyme called papain-like protease, altace indications and usage or PLpro, is one of two proteases that are required for the initial replication steps of the virus as well as silencing host immune responses, making this enzyme a sought-after drug target.
Scott Pegan, a professor of biomedical sciences in the School of Medicine at the University of California, Riverside, has led a team that investigated the PLpro from a subgroup 2b bat coronavirus, BtSCoV-Rfl.2004, to determine if identifiable trends in enzymatic activity exist within all subgroup 2b PLpros.
In a paper published in ACS Infectious Diseases, the team lays out the similarities in biochemical function among PLpros from SARS-CoV-2, SARS-CoV-1, and those of other SARS-like viruses already circulating among bats and other species. The work has revealed that unlike other types of coronaviruses, these subgroup 2b SARS and SARS-like coronaviruses seek to selectively target a specific form of ubiquitin—a small protein that exists in all eukaryotic cells—linked to key host immune pathways. Additionally, these PLpros have evolved to selectively target a ubiquitin-like protein known as ISG15 only from a subset of species.
With this information in hand, researchers can further zero in on how SARS and SARS-like viruses go undetected by the host immune system during the early stages of infection and which hosts specific coronaviruses have frequented.
“The pandemic has highlighted the urgent need to develop effective coronavirus therapeutics that can prevent current and future coronavirus subgroup 2b health threats,” Pegan said. “Our paper highlights that PLpro is not just a valid drug target for the current threat of COVID-19, but for other coronaviruses from that group that could cross from animals to humans in the future. Our work has potential to develop a therapy effective against SARS-CoV-2 and other coronaviruses lurking around the corner.”
Pegan explained that the conserved nature of PLpros among subgroup 2b coronaviruses presents an opportunity to develop inhibitors that can be used to thwart viral threats.
“Our goal is to open the door to future therapeutic design considerations for targeting PLpro as a strategy for pan-coronavirus subgroup 2b therapeutics,” Pegan said.
Pegan and his colleagues used the PLpro of BtSCoV-Rfl.2004 as a tool alongside PLpros of SARS-CoV-1 and SARS-CoV-2 to push the development boundaries of two small molecule scaffolds shown by Pegan to have antiviral properties against SARS-CoV-1 and SARS-CoV-2. This led to the design of 30 next generation drug-like subgroup 2b PLpro inhibitors that
provide new directions for pan-coronavirus subgroup 2b antiviral developments of PLpro inhibitors.
In the paper, the researchers demonstrate that these types of compounds can be pan inhibitors of PLpro and highlight their safety profiles at a cellular level.
“In particular, we push forward the development of a set of compounds from which a practical therapeutic may come,” Pegan said.
Pegan was joined in the research by several colleagues at the University of Georgia. Of these, David Crich led the compound synthesis team and served as co-designer of the compounds with Pegan; Ralph Tripp led the antiviral testing group; and Brian Cummings, now at Wayne State University, led the toxicology efforts.
Source: Read Full Article
