Can dexamethasone reduce prolonged COVID-19 symptoms?
Infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the virus responsible for the present coronavirus disease 2019 (COVID-19) pandemic, causes a wide spectrum of symptoms. Unfortunately, a minority of patients with COVID-19 require hospitalization due to progressive respiratory failure with a mortality rate of 20%.
 Study: Impact of dexamethasone on persistent symptoms of COVID-19: an observational study. Image Credit: Cryptographer / Shutterstock.com
Study: Impact of dexamethasone on persistent symptoms of COVID-19: an observational study. Image Credit: Cryptographer / Shutterstock.com
Background
Many individuals who survived the acute phase of the infection were found to suffer from prolonged symptoms that are termed ‘long Covid’ or post-acute sequelae of COVID-19 (PASC).
A large randomized trial performed at United Kingdom (U.K.) hospitals throughout the pandemic indicated that the steroid dexamethasone was capable of reducing mortality in hospitalized patients who require oxygen. Although dexamethasone is used widely, little is known about its impact on prolonged symptoms after patients have recovered from COVID-19.
A new study published on the preprint server medRxiv* assesses the symptom burden and quality of life of hospitalized patients eight months after recovering from COVID-19 by comparing the individuals before and after being treated with dexamethasone.
About the study
The current study recruited 198 patients who were admitted with COVID-19 pneumonia from a single hospital in the U.K. between April 2020 and August 2020. The inclusion criteria were either a positive polymerase chain reaction (PCR) test against SARS-CoV-2 or a clinical-radiological diagnosis of COVID-19. The patients who received steroid treatment two weeks before admission or those who were on long-term steroids were excluded from the study.
The eligible patients were divided into two groups including one group who received 6 milligrams (mg) of oral dexamethasone once daily and the control group. Both groups consisted of patients with a similar age range, frailty score, presence of comorbidities, and requirement for ventilation during admission at baseline. However, more men and a higher rate of prior chronic lung disease were reported in the control group that did not receive dexamethasone.
Patients who required oxygen following their admission were identified and grouped based on whether they have been prescribed dexamethasone upon admission. The duration of the dexamethasone course was recorded, along with routine clinical and demographic information.
All patients were followed up for eight months after they had recovered from COVID-19 either by phone or in-person visits. The patients were asked to complete a short survey that included questions on their quality of life and a review of ongoing symptoms.
Study findings
The results indicated that across the two groups, 68% of the patients reported at least one ongoing symptom eight months post-infection. The most frequently reported symptoms were fatigue, insomnia, and breathlessness. However, the patients receiving dexamethasone reported fewer symptoms as compared to the control group.
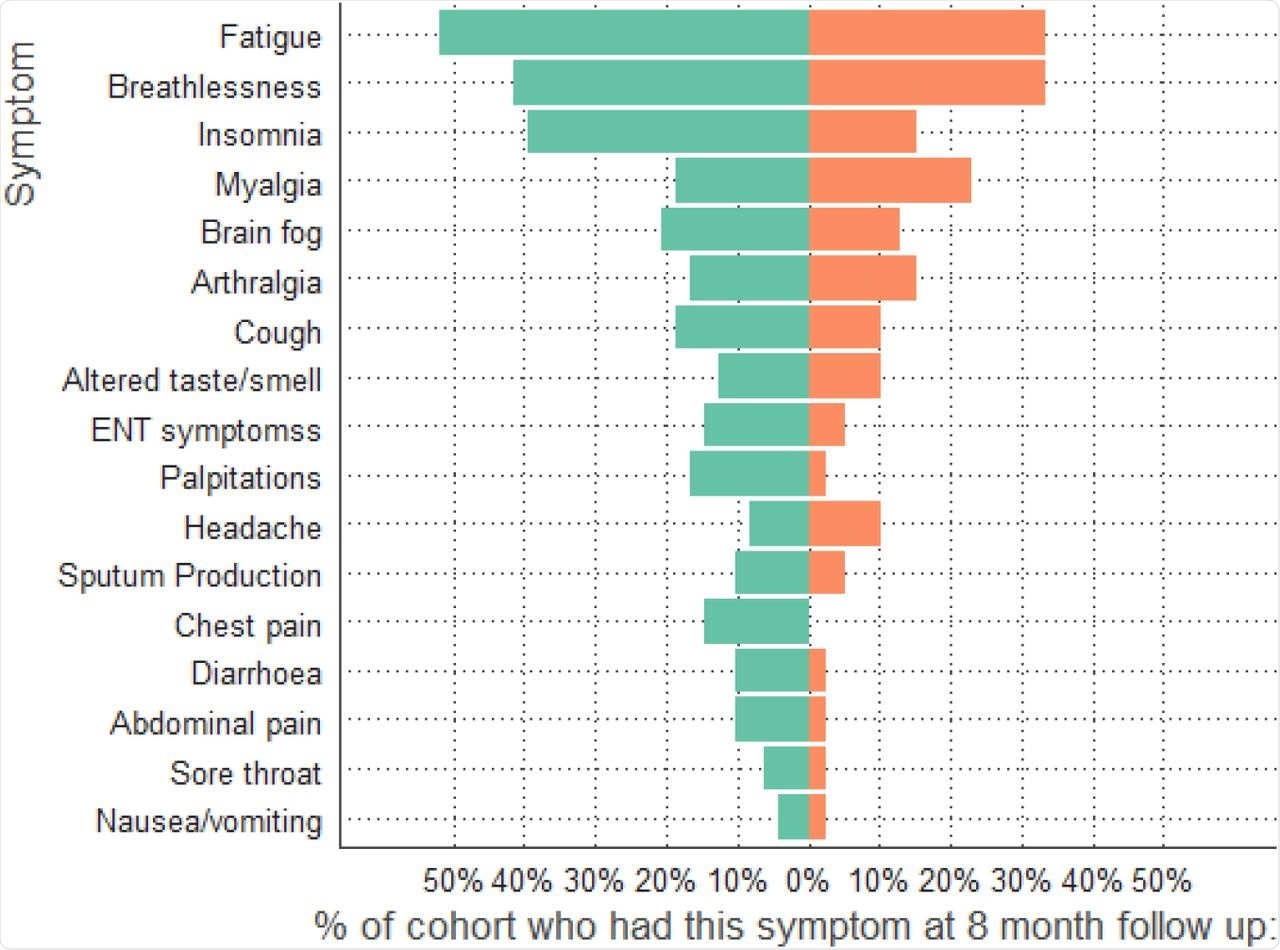 Symptoms at 8-month follow-up comparing dexamethasone group (orange) to no dexamethasone group (green).
Symptoms at 8-month follow-up comparing dexamethasone group (orange) to no dexamethasone group (green).
The mental and physical composite scores extracted from the questionnaire indicated no significant difference between the dexamethasone and control groups.
However, there can be several implications of the result. First, the patients receiving the steroid were most likely to recover, irrespective of the steroid used. Second, the use of dexamethasone was increased during the pandemic; therefore, the dexamethasone group was recruited later than the control group.
Therefore, reduced symptomatology in the dexamethasone group could be due to general improvements in care that led to a reduction of long-term symptoms. Third, the results could be due to more acute patients dying and not being available for follow-up. Fourth, the association between dexamethasone and reduced mortality might be casual.
The current study had several limitations. First, the sample size of the study was small and resulted in minimal data attrition. Second, the study is limited to only those patients who required oxygen during hospitalization. Third, the trial caused harm to hospitalized patients who did not require oxygen.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Milne, A., Maskell, S., Sharp, C., et al. (2021). Impact of dexamethasone on persistent symptoms of COVID-19: an observational study. medRxiv. doi:10.1101/2021.11.17.21266392. https://www.medrxiv.org/content/10.1101/2021.11.17.21266392v1.
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Chronic, Coronavirus, Coronavirus Disease COVID-19, Dexamethasone, Fatigue, Hospital, Insomnia, Lung Disease, Mortality, Oxygen, Pandemic, Pneumonia, Polymerase, Polymerase Chain Reaction, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Steroid, Syndrome, Virus

Written by
Suchandrima Bhowmik
Suchandrima has a Bachelor of Science (B.Sc.) degree in Microbiology and a Master of Science (M.Sc.) degree in Microbiology from the University of Calcutta, India. The study of health and diseases was always very important to her. In addition to Microbiology, she also gained extensive knowledge in Biochemistry, Immunology, Medical Microbiology, Metabolism, and Biotechnology as part of her master's degree.
Source: Read Full Article
